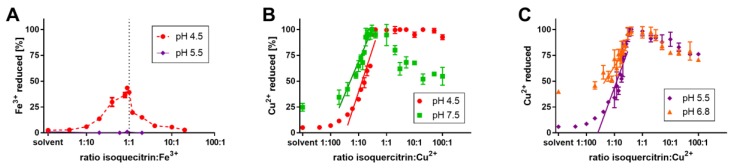
Figure 5.
Metal reduction by isoquercitrin: (A) ferric ion reduction after 5 min. Data for pH 6.8 and 7.5 are not shown since there was no reduction similarly demonstrated for pH 5.5. (B,C) cupric reduction after 5 min. Isoquercitrin in respective buffers was mixed with Fe3+ or Cu2+ ions (the final concentration of both was 50 μM) and the indicator ferrozine or BCS was added, respectively. The absorbance was measured immediately and after 5 min. The percent reduction was calculated vs. positive control sample containing the Fe3+/Cu2+ ions with hydroxylamine as the reductant. There is a linear dependence between the reduction and ratio (or concentration) in the case of cupric reduction if we neglect both poles (maximal reduction and insignificant reduction vs. solvent).