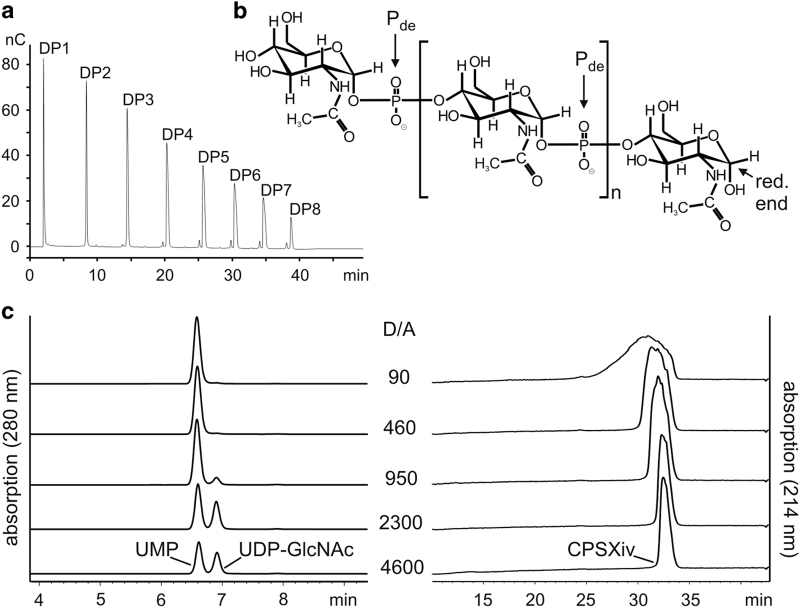
Figure 1.
The priming oligosaccharide fraction and evaluation of reaction conditions. (a) HPAEC-PAD profiling shows that the primCPSXiv pool used for upscaling the CPSXiv synthesis contains the (oligo)saccharide species DP1–DP8. The minor peaks preceeding DP3–DP8 indicate the presence of small amounts of oligomers with non-reducing end phosphate groups. (b) Chemical structure of CPSX. Pde, phosphodiester; red. end, reducing end. (c) To determine the D/A ratio that allows complete consumption of UDP-GlcNAc, CsxA reactions were carried out with a constant donor (10 mM UDP-GlcNAc) and varying acceptor (primCPSXiv) concentrations to give D/A ratios as indicated. After overnight incubation, the product spectra were analysed by HPLC-AEC. Although a small peak indicating residual UDP-GlcNAc was visible at the D/A ratio 950, conversion to UMP was complete at D/A ratio 460. CPSX, NmX capsule polysaccharide; CPSXiv, in vitro produced CPSX; HPAEC-PAD, high-performance anion-exchange chromatography with pulsed amperometric detection; HPLC-AEC, high-performance liquid chromatography based anion exchange chromatography.