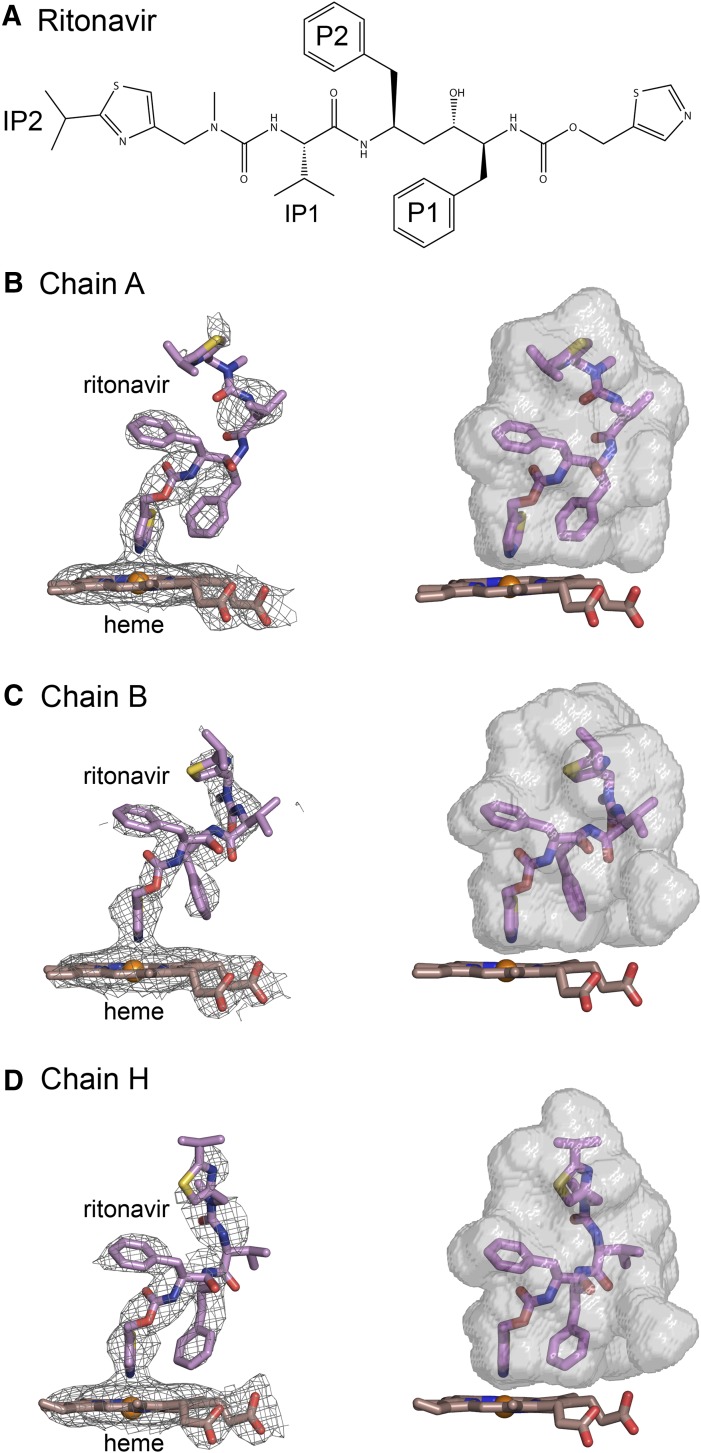
Fig. 2.
(A) Structure of ritonavir. (B–D) Composite omit 2mFo-DFc maps contoured at 1 σ around the heme and ritonavir are shown on the left side, and surfaces for the substrate binding cavity are rendered in relation to the heme and ritonavir on the right for chains A, B, and H, respectively. The conformations of ritonavir and the shapes of the cavities vary between chains. The nitrogen of the thiazole group is coordinated to the heme with bond distances of 2.20, 2.16 and 2.27 Å, respectively for chains A, B, and H. Carbons are colored violet for ritonavir and brown for the heme. Sulfur is colored yellow. Other atom colors are defined in the legend to Fig. 1. Fc, calculated structure factor; Fo, observed structure factor.