Fig. 4.
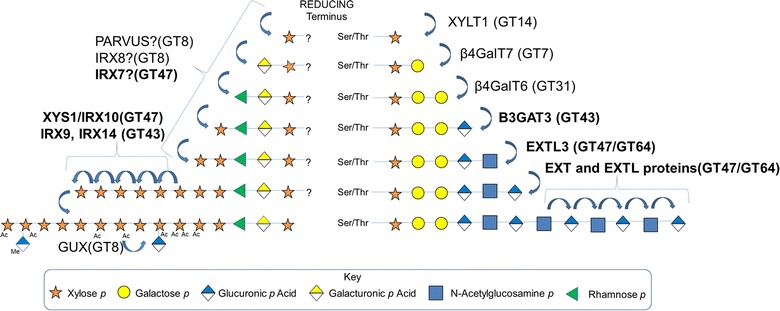
Models of glucuronoxylan and heparan sulfate biosynthesis. Comparison of proposed models of xylan and heparan sulfate biosynthesis. In bold are enzymes from the families’ common between the two pathways (GT43 and GT47). In heparan sulfate biosynthesis, polysaccharide initiation occurs by the transfer of a xylosyl residue to a protein serine or threonine residue by the enzyme xylosyl transferase 1 (XYLT1) [77]. A linker tetrasaccharide is then synthesized by the enzymes β-1-4 galactosyl transferase 7(β4GalT7), β-1-4 galactosyl transferase 6(β4GalT6) and a GT43 family enzyme Galactosylgalactosylxylosylprotein 3-β-glucuronosyltransferase 3(β3GAT3). Following primer synthesis, the polymer is extended by the GT47/64 heparan synthases, exotosin (EXT) and exotosin-like (EXTL3) proteins, which catalyze the transfer of the repeating segment of glucuronic acid (GlcAp) and N-acetyl glucosamine (GlcNAcp) [77]. This mechanism has similarities to our proposed model for xylan synthesis, where a tetrasaccharide primer may be synthesized while connected to some unknown carrier in the ER/Golgi, potentially in part by GT47 and GT43 family enzymes. This primer is then extended by the GT47 XYS1/IRX10 family of proteins, which most likely function as part of protein complexes that also contain members of GT43 (IRX9, IRX14). The xylan chains are then decorated with sidechains such as acetyl esters and glycosyl units such as (Me) GlcAp
