Table 1. Optimization of substrates and reaction conditions for the synthesis of 1A.
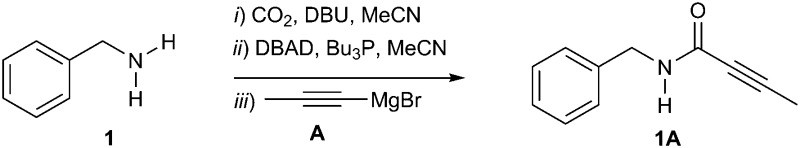
| ||||
| Entry a | DBU (equiv.) | DBAD (equiv.) | Bu3P (equiv.) | Yield of 1A b (%) |
| 1 | 0.1 | 2 | 2 | 0 |
| 2 | 0.1 | 3.8 | 3.8 | 0 |
| 3 | 0.2 | 3.8 | 3.8 | 0 |
| 4 | 0.05 | 1 | 1 | 0 |
| 5 | 0.05 | 2 | 2 | 46 ± 8 c |
| 6 | 0.05 | 3.8 | 3.8 | 44 d |
| 7 | 0.05 | 7.2 | 7.2 | 0 |
aReaction conditions: CO2 was bubbled in a solution of 1 (138.6 μmol, 1.0 equiv.), DBU (0.05–0.2 equiv.) in MeCN (1 mL), r.t. for 40 min. Mitsunobu reagents (7.2–1 equiv.) in MeCN (0.5 mL) were added and the solution stirred for 10 min. A (7.2 equiv. of a 0.5 M solution in THF) was added and quenched after 30 min.
bYield of isolated 1A calculated from compound 1.
c N = 4.
d N = 1.
