Abstract
Both recovery time of post-exercise muscle oxygenation and muscle strength decline with aging. Although beetroot consumption has been shown to improve muscle oxygenation and exercise performance in adults, these effects in the elderly has not been addressed. The aim of the present study was to evaluate the effect of a beetroot-based gel (BG) on muscle O2 saturation, blood volume (tHb) and handgrip strength in the elderly in response to handgrip exercise. In a randomized crossover double-blind design, twelve older subjects consumed BG (100 g of beetroot-based gel containing ~ 12 mmol nitrate) or PLA (100 g of nitrate-depleted gel nitrate-depleted). The subjects performed a rhythmic handgrip exercise which consisted of a one 1-min set at 30% of the maximal voluntary contraction (MVC) of each subject, followed by a 1 min recovery. The muscle oxygenation parameters and tHb were continuously monitored by using near-infrared spectroscopy. MVC was evaluated at baseline, immediately after exercise, and 30 min afterwards. The muscle O2 resaturation rate during exercise recovery was greater in the BG when compared to PLA condition (1.43 ± 0.77 vs 1.02 ± 0.48%.s-1; P < 0.05). Significant increase was observed in tHb during exercise recovery (10.25 ± 5.47 vs 6.72 ± 4.55 μM; P < 0.05) and significant reduction of handgrip strength decline was observed 30 min after exercise in BG (- 0.24 ± 0.18 vs—0.39 ± 0.20 N; P < 0.05). In summary, a single dose of a beetroot-based gel speeds up muscle O2 resaturation, increases blood volume and improves recovery of handgrip strength after handgrip exercise in older adults.
Introduction
Aging causes numerous changes to the cardiovascular system including increased total peripheral resistance and vascular endothelial dysfunction, which has been associated with the reduced bioavailability of nitric oxide (NO) [1]. Since NO may influence O2 utilization by the cells, this suggests that O2 delivery to the working muscle during exercise may be compromised in the elderly.
Muscle O2 saturation (SmO2) measured by near-infrared spectroscopy reflects the balance between muscle O2 delivery and muscle O2 utilization during exercise and/or during the recovery time after exercise [2]. Recovery time of muscle O2 resaturation following the completion of exercise has been used as an index of deficient muscle O2 delivery in relation to muscle O2 demand [3,4]. Kutsuzawa et al. [3] demonstrated that recovery rate of muscle O2 resaturation after submaximal handgrip exercise was delayed with increased age. Ichimura et al. [4] demonstrated that the half-recovery time of oxygenated hemoglobin/myoglobin was significantly related to age, since it was greater in the elderly subjects than in the middle-aged subjects. These data suggest that age-related prolongation in recovery time of muscle O2 resaturation reflects the age-related decline in muscle function, which is dependent on O2 delivery to working muscles.
Recently, the consumption of beetroot, a food rich in nitrate, has gained popularity in scientific literature due to the possible effect of the nitrate present in this food to promote NO bioconversion [5,6]. Furthermore, short-term beetroot consumption has been demonstrated to improve muscle oxygenation status during moderate to severe exercise in healthy adults [7,8,9,10]. Whether beetroot consumption has the ability to increase muscle O2 delivery and/or increase the capacity of muscle to extract or utilize O2 in older adults has not been determined. For this reason, the aim of the present work was to evaluate whether beetroot-based nutritional gel rich in nitrate promotes changes in the SmO2 parameters, blood volume and muscle strength of the forearm following the completion of a handgrip exercise protocol. Analysis of urinary nitrate and nitrite were also addressed. We hypothesized that a single dose of the beetroot gel rich in dietary nitrate would result in increased NO bioconversion and would, consequently, promote increases in the muscle oxygenation, blood volume and forearm strength after handgrip exercise in the elderly.
Materials and methods
Participants
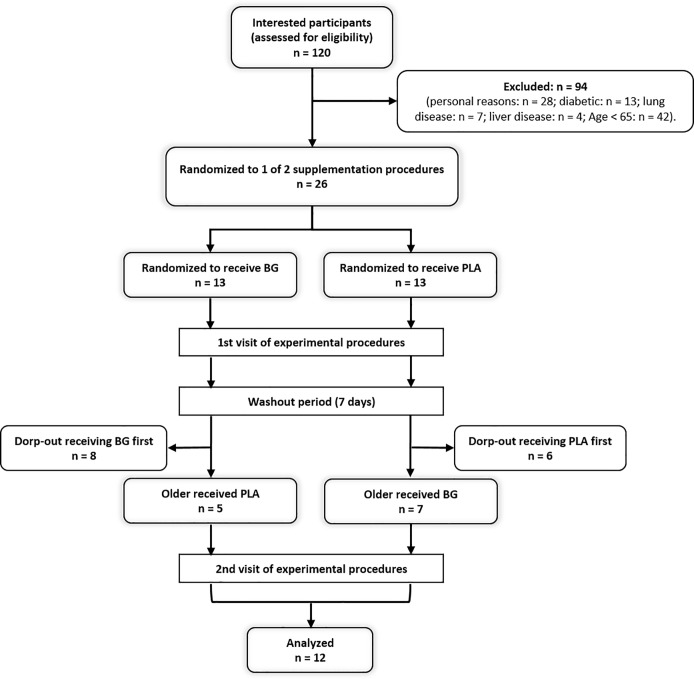
One hundred twenty individuals were recruited through announcements in flyers and advertisements during community events at Family Center of the Elderly in Macaé, Rio de Janeiro, Brazil. Twenty-six elderly individuals were eligible to participate in the study and twelve completed all the experimental procedures (Fig 1). An a priori power analysis was conducted (G*Power v. 3.0.1) for an F test (repeated measures, within-between interaction for two time points). On the basis of a statistical power (1 – β) of 0.80, a moderately large effect size (0.5), and an overall level of significance of 0.05, at least 12 participants were needed to detect a statistical difference. Exclusion criteria included people aging < 65 years, smoking, beetroot allergy, unwillingness to avoid beetroot products during the entire study, liver and kidney disease, diabetes, and/or acutely ill. All eligible participants were fully informed of the nature and purpose of the investigation and provided written consent to participate. All experimental procedures were performed in accordance with the ethical standards of the Declaration of Helsinki and approved by the institutional ethics committee and the trial was registered in the ClinicalTrials.gov (NCT02772900).
Fig 1. Flow diagram of the progress through the phases of the randomized, crossover and placebo-controlled study.
Study design
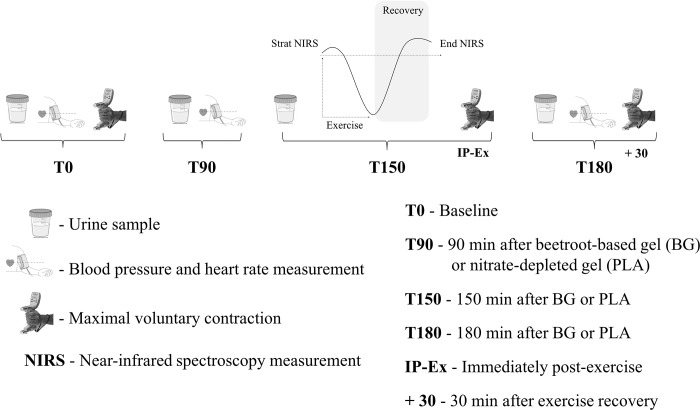
The study was conducted in a randomized, double-blind, crossover and placebo-controlled way conducted from April 2016 to January 2017. All participants reported to the laboratory on three occasions, with at least 1-week interval between visits. The first visit was used to explain the experimental procedures and collect clinical and anthropometric data, as well as to ensure familiarity with the handgrip exercise protocol. In the second and third visits urine samples were drawn at baseline (T0) after a 10-min period of quiet rest. Thereafter, subjects were randomly divided into either a beetroot-based nutritional gel (BG) or a control nitrate-depleted gel (PLA). NIRS measurements in response to the handgrip exercise protocol began approximately 150 min after the nutritional intervention (T150) and lasted for approximately 10 min. Urine samples were drawn again at T90, T150 and T180 after nutritional intervention (Fig 2). The three visits were held between 07:00 and 12:00 a.m. The participants were instructed to fast for at least 8 hours before each visit, restrict carbonated mineral water, avoid the intake of foods rich in nitrate and nitrite, and not to use any mouthwash.
Fig 2. Experimental design of the study.
Nutritional intervention
All subjects were orally administered either 100 g of a beetroot-based nutritional gel (BG) or a nitrate-depleted gel (PLA) in identical forms in a double-blind and randomized manner. After 5 minutes of complete consumption of the gel, 400 mL of deionized water was provided to the subject in order to eliminate any residual gel in the mouth. The content of nitrate of the BG and PLA consumed by the subjects of the present study were 12.2 ± 0.18 and 0.02 ± 0.02 mmol/100g, respectively. The BG and PLA were prepared in our laboratory according to the procedures Oliveira et al. [11] and Morgado et al. [12]. Briefly, in order to produce 100 g of the beetroot-based gel, ~16.2 g of the beetroot powder was mixed with ~80 ml of BJ, ~2.8 g of carboxymethylcellulose and ~1ml of artificial orange flavor, and mixed until it obtained the consistency of a gel. These steps last approximately ~30 min. For nitrate-depleted gel production there was an additional three-hour preparation time for the removal of nitrate from beetroot. We have chosen to use a beetroot-based gel product in the present study since the consistency of the beetroot-based gel is likely to increase the amount of time and mouth contact associated with the intake of nitrate, which may increase the ability of the nitrate present in the beetroot to be reduced to nitrite in the oral cavity by the enzymatic action of nitrate reductase via oral commensal bacteria.
Urinary nitrite and nitrate analysis
Nitrite and nitrate analyses in urine were performed as previously described by Croitoru [13], using a high-performance liquid chromatography (HPLC) system. Nitrite is a metabolic end-product derived from oxidation of NO and was used in the present study as an indicator of NO bioconversion/bioavailability [6]. Measurements were made at T0, T90, T150 and T180. Sodium nitrite and nitrate was used for quantification, and the results were expressed as mmol.mmol-1 creatinine for nitrate and μmol.mmol-1 creatinine for nitrite. On the day before each visit, the participants were instructed to avoid foods rich in nitrate and nitrite in their diet.
Muscle oxygenation and blood volume measurements
Forearm SmO2 of the flexor muscle (flexor carpi radialis) of each subject’s dominant forearm was continuously monitored using a commercially available portable NIRS device (PortaMon, Artinis, Medical Systems). Portable NIRS had 3 transmitters and one receiver. For congruence in measurement depth, the inter-optode distance of 35 mm was used for further analysis of the concentration changes in oxyhaemoglobin (O2Hb) and deoxyhemoglobin (HHb). This device is a 2-wavelength, continuous wave system, which simultaneously uses the modified Beer-Lambert law and spatially resolved spectroscopy (SRS) methods [14]. The portable NIRS device was placed on the skin, over the dominant forearm muscle, exactly 5 cm distal of the medial epicondyle of the humerous. Forearm skin-fold thickness (9.0 ± 8.7 mm) of each subject was less than the penetration depth (approximately 15 mm) of the portable NIRS device that is half of the inter-optode distance (35 mm), ensuring that measurements were recorded from the forearm muscle tissue [15]. Measurement of the skin-fold thickness was grasped from proximal forearm, in anteromedial aspect of the flexor muscles of the forearm, approximately 5 cm distal of the medial epicondyle of the humerous. The skin-fold was raised vertically with the thumb and index finger of the left hand and the forearm skin-fold was measured using a Lange Skin-fold Caliper. To secure the probe on the skin and minimize movement during exercise, an elastic bandage was wrapped around the subject’s forearm. The wrap also helped to minimize the possibility that extraneous light could influence the signal. During all tests, the NIRS system was connected to a personal computer via Bluetooth for data acquisition (10Hz), analogue-to-digital conversion and subsequent analysis of the raw data (i.e. no filter was used) using native software (Oxysoft version 2.1.6; Artinis Medical Systems). The software calculates changes in light absorption at the different wavelengths (750 and 850 nm) and converts them to relative concentrations of O2Hb and HHb using the modified Lambert law to correct for light scattering within the tissue. A fixed value of 4 for the differential path-length factor (DPF) was used to calculate absolute difference changes [16,17]. Blood volume (tHb) was calculated as O2Hb + HHb and SmO2 was calculated as [O2Hb/tHb]*100 using the spatially resolved spectroscopy method [18,19].
The following NIRS parameters were considered for statistical analysis: 1) SmO2 and tHb at baseline (Pre), 1, 5, 10, 20, 30, 40, 50 and 60 seconds of exercise and exercise recovery. SmO2 and tHb were reported as changes from baseline (30 s prior to exercise) and each time point (i.e.: Pre, 1, 5, 10, 20, 30, 40, 50 and 60 seconds) was converted to seconds by calculating the average of their respective time in milliseconds during exercise and exercise recovery (e.g.: the first second was calculated by the average of the first 60 milliseconds of data acquisition during exercise and exercise recovery, the fiftieth second was calculated by the average of the first 300 milliseconds of data acquisition during exercise and exercise recovery, and so on…); 2) Minimum SmO2 (SmO2min), which corresponds to the minimum SmO2 values within 60 s the exercise; 3) ΔSmO2 half-desaturation time (ΔSmO2½DT), which corresponds to the period of time between the start of the exercise until SmO2 reaches 50% of the difference between baseline SmO2 and the minimum SmO2 during the exercise; 4) SmO2 desaturation rate (SmO2DR), which corresponds to the downslope of SmO2 during the exercise; 5) SmO2 resaturation rate (SmO2RR), which corresponds to the upslope of SmO2 during the exercise recovery; and 6) Exercise and exercise recovery tHb amplitude (ΔtHbEx and ΔtHbRec, respectively) was identified as the difference between the maximum and minimum tHb values within exercise and exercise recovery period.
Forearm muscle strength
Dominant handgrip strength was measured by using an adjustable, hydraulic handgrip dynamometer (JAMAR, Model 5030J1, Sammons Preston Roylan, Bolingbrook, IL, USA). The procedures of the handgrip strength measurement were performed as described by Beam and Adams [20]. Three repetitions were performed with 30 s of rest between repetitions at baseline, immediately after the handgrip exercise protocol (IP-Ex), and approximately 30 min after exercise (+30). Each attempt was terminated when force showed a clear stagnation or a decrease. The maximal voluntary contraction (MVC) force was recorded in kg and converted to newtons by multiplying the kg value by 9.8067. The strength values were expressed by the difference with baseline values (in newtons) and adjusted to each subject’s body weight.
Exercise protocol
The exercise consisted of rhythmic contractions of their forearm muscles at a controlled rate (60 contractions per minute, 0.5-s contraction/0.5-s relaxation) and range of motion (10-cm excursion of the pulley wire with each contraction). Each subject completed one 1-min bout of exercise at 30% of the MVC of each subject, with 1 min of quiet recovery. Subjects were in a supine position and used the dominant arm to perform the exercise.
Statistical analysis
The normality, homogeneity of variances and sphericity of the data were examined with the Shapiro-Wilk, Levene and Mauchly tests, respectively. To identify differences in the muscle O2 saturation parameters (SmO2DR and SmO2RR) and blood volume (ΔtHbEx and ΔtHbRec) between BG and PLA interventions, a Paired t-test was used. When the assumption of normality and homogeneity of variances were violated, a Wilcoxon test was used. To identify differences in SmO2, tHb, forearm muscle strength, urinary nitrite and nitrate between BG and PLA, a two-way ANOVA with repeated measures on one factor (time) was used. In addition, to verify whether the order of BG or PLA condition affected the results, ANOVA with repeated measurement was also performed in this study. When a significant F was found, additional post-hoc tests with Bonferroni adjustment were performed. When the assumption of normality and homogeneity of variances were violated, a Friedman Test followed by post hoc analysis with Wilcoxon signed-rank tests and a Bonferroni adjustment were used. For all variables, when sphericity was violated, a Greenhouse-Geisser correction was used. Statistical significance was set at the 0.05 level of confidence. All analyses were performed using a commercially available statistical package (IBM SPSS Statistics version 23 for Mac, Chicago, IL), and the results were expressed as means ± standard deviation (SD).
Results
The assumption of normality, homogeneity of variance and sphericity were violated for SmO2 and tHb during exercise. In addition, for SmO2DR the assumption of normality was violated. Therefore, a non-parametric test was used for statistical analysis of these variables. Sphericity was violated for urinary nitrate and nitrate. For all other variables, the assumptions of normality, homogeneity of variances and sphericity were not violated. The plot of each residuals analysis of SmO2 and tHb during exercise and recovery period for both BG and PLA conditions was included as Supplementary Materials (S1 File).
Of the twenty-six eligible participants (100%) in the randomization phase of the study, twelve (three men and nine women) (46%) completed the study. Among the 14 participants that declined (54%), eleven participants withdrew for personal reasons and three participants withdrew due to illness unrelated to the study. Among the personal reasons, the discomfort of the exercise protocol and the duration of each experimental procedure visit (which lasted approximately 4 hours each) were the most stated by the participants who declined. The baseline volunteers’ characteristics are shown in Table 1.
Table 1. Baseline characteristics of the subjects completing the study.
| n (female) | 12 (9) |
| Age (years) | 68.8 ± 3.5 |
| Body mass (kg) | 70.4 ± 8.7 |
| BMI (kg/m2) | 29.3 ± 3.0 |
| Waist circumference (cm) | 97.5 ± 9.0 |
| MVC (N) | 26.5 ± 5.79 |
The values are mean ± SD. BMI = Body mass index, MVC = maximal voluntary contraction.
Urinary nitrite and nitrate analysis
The urinary concentrations of nitrite and nitrate before and after BG and PLA interventions are shown in Table 2. There was no difference in urinary nitrate (P = 0.813) and nitrite (P = 0.654) between BG and PLA interventions at T0. There was a significant main effect regarding time for nitrate (P = 0.006) and nitrite (P = 0.007). Post hoc tests revealed that urinary concentrations for nitrate increased more from T0 to T90 (P = 0.028), T150 (P = 0.004) and T180 (P = 0.000) and for nitrite increased more from T0 to T90 (P = 0.030), T150 (P = 0.028) and T180 (P = 0.037) in BG than in PLA. Furthermore, there was a significant interaction effect regarding time per treatment for urinary concentrations of both nitrate (P = 0.029) and nitrite (P = 0.038). Post hoc tests revealed that urinary concentrations for nitrate increased at T150 (P = 0.020) and T180 (P = 0.001) and for nitrite increased at T150 (P = 0.042) and T180 (P = 0.032) in BG compared with PLA intervention. In addition, there was no effect of the supplementation order (BG → PLA vs. PLA → BG) for nitrate (P = 0.564) and nitrite (P = 0.601).
Table 2. Values of nitrate and nitrite in both beetroot-based gel and nitrated-depleted gel conditions.
| Variable | Condition | T0 | T90 | T150 | T180 |
|---|---|---|---|---|---|
| Nitrate (mmol.mmol creatinine-1) | BG | 0.01 ± 0.01 | 0.12 ± 0.11a | 0.21 ± 0.16a,* | 0.21 ± 0.12a,* |
| PLA | 0.01 ± 0.00 | 0.04 ± 0.02 | 0.05 ± 0.04 | 0.02 ± 0.02 | |
| Nitrite (mmol.mmol creatinine-1) | BG | 0.98 ± 1.01 | 2.13 ± 1.45a | 5.33 ± 6.64a,* | 4.66 ± 5.81a,* |
| PLA | 0.83 ± 0.52 | 1.36 ± 0.71 | 1.55 ± 0.89 | 1.10 ± 0.79 |
The values are mean ± SD. BG = beetroot-based gel, PLA = nitrate-depleted gel.
* Significantly different from PLA.
a Significantly different from T0. Statistical significance was set at the 0.05 level of confidence based on post hoc test with Bonferroni adjustment.
Muscle oxygenation and blood volume
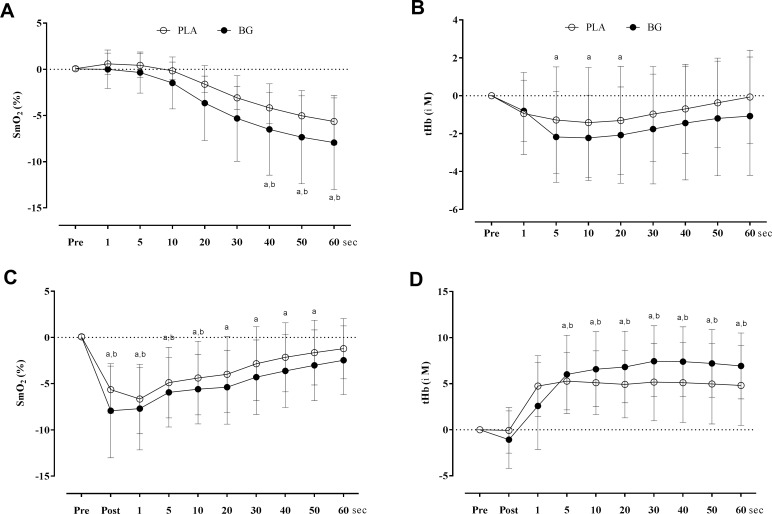
Changes in SmO2 and tHb during the exercise and exercise recovery are depicted in Fig 3. Values of the SmO2min, ΔSmO2½DT, SmO2DR and SmO2RR, ΔtHbEx and ΔtHbRec are presented in the Table 3. SmO2RR was greater (P = 0.043) in BG as compared to PLA condition and ΔtHbRec was significantly higher (P = 0.050) in BG as compared to PLA. Furthermore, there was a significant decrease in SmO2min (P = 0.043) in BG as compared to PLA condition. There was a significant main effect regarding time for SmO2 (P = 0.000) during exercise. Post hoc tests revealed that SmO2 decreased more from T0 to 20 (P = 0.019), 30 (P = 0.002) and 40 (P = 0.001) seconds of exercise in BG than T0 to 20 (P = 1.000), 30 (P = 0.237) and 40 (P = 0.052) in PLA, and decreased more from T0 to 50 and 60 seconds of exercise in both BG (P = 0.000 and P = 0.000, respectively) and PLA (P = 0.017 and P = 0.008, respectively). Significant increase in tHb at 5, 10, 20, 30, 40, 50 and 60 seconds of exercise recovery was observed in both BG and PLA as compared to baseline values (main effect for time, P = 0.000). No significant differences were observed in tHb (interaction effect for time by treatment, P = 0.123) and SmO2 (interaction effect for time by treatment, P = 0.650) at any time point during exercise recovery, and ΔSmO2½DT (P = 0.299) between both BG and PLA conditions. There was no effect of the supplementation order (BG → PLA vs. PLA → BG) for SmO2 during exercise (P = 0.759), SmO2 during recovery (P = 0.130), SmO2min (P = 0.286), ΔSmO2½DT (P = 0.337), ΔSmO2½RT (P = 0.440), ΔtHbEx (P = 0.598) and ΔtHbRec (P = 0.060). The relative changes in oxyhaemoglobin (O2Hb) and deoxyhemoglobin (HHb) during exercise and recovery period for both BG and PLA conditions was included as Supplementary Materials (S1 and S2 Tables).
Fig 3.
Values of muscle oxygenation (ΔSmO2, %) and blood volume (ΔtHb, μM) during (A and B, respectively) and following (C and D, respectively) the rhythmic handgrip exercise after beetroot-based nutritional gel (BG) and nitrate-depleted gel (PLA) intervention. Pre = baseline; Post = post-exercise. a significantly different from Pre for BG and b significantly different from Pre for PLA.
Table 3. Changes in muscle oxygenation and blood volume parameters.
| BG | PLA | |
|---|---|---|
| SmO2min (%) | - 13.68 ± 6.77* | - 10.70 ± 5.02 |
| ΔSmO2½DT (s) | 15.92 ± 7.25 | 18.42 ± 7.48 |
| SmO2DR (%.s-1) | -1.90 ± 0.94 | -1.72 ± 0.88 |
| SmO2RR (%.s-1) | 1.43 ± 0.77* | 1.02 ± 0.48 |
| ΔtHbEx (μM) | 10.48 ± 5.83 | 9.99 ± 3.39 |
| ΔtHbRec (μM) | 10.25 ± 5.47* | 6.72 ± 4.55 |
Values are expressed as means ± SD. BG = beetroot-based nutritional gel, PLA = nitrate-depleted gel, SmO2 = muscle O2 saturation, tHb = blood volume.
* Significantly different from PLA.
Forearm muscle strength
Changes from baseline in maximal voluntary contraction (ΔMVC) of the forearm muscle immediately after exercise (IP-Ex) and 30 minutes of recovery (+30) in the BG and PLA conditions are presented in Fig 4 and Table 4. No significant difference between BG and PLA conditions were observed for MVC at T0 (P = 0.982). There was a significant decrease in MVC from T0 to IP-Ex in both BG and PLA condition (main effect for time, both P = 0.000). There was a significant reduction in ΔMVC at +30 in the BG when compared to PLA condition (interaction effect for time by treatment, P = 0.050). No effect of the supplementation order (BG → PLA vs. PLA → BG) for MVC was observed (P = 0.116).
Fig 4. Changes from baseline in maximal voluntary contraction (ΔMVC, N) of the forearm muscle immediately after exercise (IP-Ex) and 30 minutes of recovery (+30) after beetroot-based nutritional gel (BG) and nitrate-depleted gel (PLA) intervention.
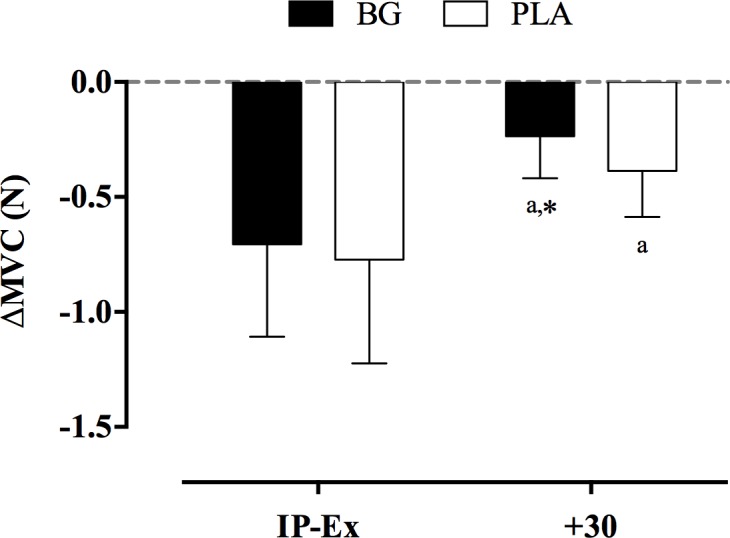
The symbol*(P < 0.05) denotes significantly different from PLA and a significantly different from T0.
Table 4. Absolute values of maximal voluntary contraction (MVC) and difference (ΔMVC) from baseline (T0) immediately after exercise (IP-Ex) and 30 minutes of recovery (+30) after beetroot-based nutritional gel (BG) and nitrate-depleted gel (PLA) intervention.
| Variable | Condition | T0 | IP-Ex | +30 |
|---|---|---|---|---|
| MVC (N) | BG | 3.69±0.90 | 2.99±0.71a | 3.46±0.80* |
| PLA | 3.69±0.81 | 2.91±0.59a | 3.30±0.68 | |
| ΔMVC (N) | BG | - | -0.71±0.40 | -0.24±0.18b,* |
| PLA | - | -0.77±0.45 | -0.39±0.20b |
The values are mean ± SD.
The symbol*(P < 0.05) denotes significantly different from PLA.
The letters a denotes significantly different from T0 and
b denotes significantly different from IP-Ex.
Discussion
The aim of this study was to examine the effects of a single dose of a beetroot-based nutritional gel, which is rich in nitrate, on forearm muscle O2 saturation, on blood volume and forearm strength following the completion of a handgrip exercise protocol in the elderly. This is the first investigation into the effects of the single dose of beetroot-based nutritional gel on SmO2 parameters (forearm muscle O2 desaturation [ΔSmO2min, SmO2DR and ΔSmO2DT] and O2 resaturation rate (SmO2RR) and blood volume. The major findings of this study are that a single dose of a beetroot-based nutritional gel rich in nitrate increase muscle O2 extraction (SmO2min), speeds up muscle O2 resaturation rate (SmO2RR) during exercise recovery and speeds up the force recovery of handgrip strength after 30 minutes of exercise recovery. These results were associated with an increase in the muscle blood volume (ΔtHbRec) and urinary nitrite and nitrate, which increased ~3.5-fold and ~4.0-fold, respectively after 150 minutes and ~4.0-fold and ~10.0-fold after 180 minutes, respectively in BG as compared to PLA. Others studies have observed similar increases in plasma nitrite and nitrate 180 minutes after beetroot juice consumption [21,22], demonstrating that plasma nitrate and nitrite have a similar kinetic effect as observed in the urine nitrite and nitrate of the present study.
Collectively, these findings suggest that the beetroot gel promotes NO bioconversion, which may increase blood and oxygen delivery to active muscle and thereby speed up the force recovery of handgrip strength.
NO bioavailability is compromised in the systemic circulation and in the musculature of sedentary ageing humans due to increased oxidative stress [23]. In the current study, significant increases in urinary nitrate and nitrite concentrations were observed after approximately 2.5 h after BG when compared to PLA condition. These finding support the results of other study where significant increases were also observed in older people after a single dose of BG [11] and may suggest that there was absorption and a possible bioconversion of the dietary nitrate present in the BG into nitrite and, subsequently NO.
An association between ageing and reduced blood flow and O2 delivery to the active muscle in aged subjects has been documented [24,25]. Although a myriad of factors (e.g.: prostacyglin and endotelin-1) that act regulating smooth vascular muscle tonus are impaired in elderly [26], the mechanisms underlying the attenuated local vasodilatory response can be explained in part by impaired NO availability [25]. NO may influence O2 utilization by the cells, suggesting that O2 delivery to the working muscle during exercise may be compromised in the elderly. In this scenario, improved NO bioconversion would likely increase blood flow and O2 delivery to the contracting muscles. Ichimura et al. [4] observed prolongation of muscle oxygenation in aging people, suggesting a decline in muscle oxidative capacity and impairs peripheral endothelial function in this population. In the present study, SmO2RR was faster (P = 0.043) in BG as compared to PLA, along with a more pronounced blood volume increase during recovery (ΔtHbRec), which indicates a greater O2 delivery relative to O2 consumption. However, no significant difference in SmO2 was observed at the end of the 60 seconds of exercise recovery, which suggests that BG may improve O2 delivery only in the initial period of exercise recovery (~10 sec). Previous studies have also demonstrated improvements in muscle oxygenation status during moderate to severe exercise in healthy adults after beetroot consumption [7,8,9,10]. This beneficial effect is probably at least partly explained by the improved bioconversion of the dietary nitrate present in the BG into NO, which have an important role in modulating vascular tone and blood perfusion. We recently demonstrated that there was an improved endothelium-dependent vasodilation after a single dose of beetroot based gel in the elderly with cardiovascular risk factors [11]. Endothelium-dependent vasodilation has been used as a physiological measurement for evaluating NO production [27]. Therefore, this finding corroborates a possible effect of beetroot on bioconversion of NO, as encountered in the present study.
Ageing has been associated with a reduced muscle O2 extraction during exercise [23], which may limit maximal oxygen consumption and contribute to reduced muscle energy capacity [28]. Although no significant difference was observed in ΔSmO2½DT (P = 0.299) and SmO2DR (P = 0.374) in the present study, there was a significant decrease in SmO2min (P = 0.043) and SmO2 during the handgrip exercise in BG as compared to PLA condition. The lesser SmO2min observed in BG, together with no significant change in ΔtHb during exercise, may indicate a greater muscle O2 consumption of exercising muscle. This finding suggest that beetroot consumption may improve mitochondrial function in older people. Previous in vitro study has demonstrated that beetroot induce mitochondrial biogenesis and modestly increases basal cellular respiration without affecting respiratory capacity and proton leak [29]. Larsen et al. [30] demonstrated that nitrate supplementation increased mitochondrial respiratory control ratio and P/O ratio during submaximal ADP stimulation in isolated mitochondria of muscle tissue of healthy adults, indicating a better coupling between respiration and oxidative phosphorylation, and an improved mitochondrial efficiency, respectively. The effect of beetroot on the mitochondria may be mediated partially by the bioconversion of the nitrate present in this food into nitrite and NO, which has been demonstrated to be a key messenger to regulate mitochondrial number and function in various cell types [31,32]. The ΔSmO2½DT and SmO2DR represents a O2 consumption rate immediately after beginning the exercise. The absence of the effect observed in these variables may be related to muscle metabolic condition (i.e. changes in pH and O2 pressure). For example, nitrite-NO bioconversion is favored in fatiguing muscle during exercise and perhaps no differences were shown in ΔSmO2½DT and SmO2DR because muscle fatigue in the beginning of the exercise was not sufficient to generate adequate muscle metabolic condition for nitrite-NO bioconversion.
A previous study in healthy, moderately active subjects observed that a higher magnitude of deoxygenation during isometric exercise (oxidative capacity) shows slower time for muscle reoxygenation during exercise recovery, since O2 supply is not fully increased despite the increased availability of O2 [33]. Although improved muscle oxidative capacity during handgrip exercise after BG consumption (lesser SmO2min) has been observed, there was a faster O2 resaturation rate during the exercise recovery (greater SmO2RR), which suggests that BG consumption improves muscle oxidative capacity and O2 supply in the elderly. In sedentary healthy subjects, muscle reoxygenation rate during bicycle ergometer exercise recovery has been shown to be approximately 22% faster in middle-age subjects when compared with the elderly [4]. Additionally, the muscle reoxygenation rate during exercise recovery was approximately 35% faster in physically active when compared with sedentary elderly, and 37% faster in physically active when compared to sedentary middle-age subjects [4]. Our results have demonstrated that SmO2RR during the handgrip exercise recovery was 40% faster after BG when compared with PLA condition in the sedentary elderly. Hence, it is important to point out that habitual physical activity and BG condition, albeit single dose consumption, seems speed up of muscle O2 resaturation rate during exercise recovery.
Another important finding from this study is that BG promoted faster recovery from the decrease in maximal isometric strength observed after handgrip exercise. Good handgrip strength in older people is important for successful performance in activities of daily living and occupational activities. The energy pathway predominantly involved in the MVC is the phosphagen system. Because the need for large amounts of adenosine triphosphate (ATP) is so urgent, the ATP and phosphocreatine (PCr) must be immediately available for the interacting muscle filaments–actin and myosin. A possible explanation for the effect of BG in speeding up force recovery of handgrip strength could be increased resynthesis of PCr and ATP. Previous studies have reported a positive association between faster muscle O2 resaturation measured by NIRS and faster PCr resynthesis after exercise [34,35,36,37]. McCully et al. [38] simultaneously measured and PCr recovery after submaximal exercise in normal subjects using NIRS and phosphorus magnetic resonance spectroscopy, respectively, and found that the time constants of these indices were similar. The authors suggested that the muscle O2 resaturation is rate limiting for ATP synthesis, when evaluated as the rate of PCr recovery after submaximal exercise. Furthermore, nitrate increases the rate of human skeletal muscle PCr recovery after exercise in hypoxia, suggesting an augmented maximum rate of oxidative ATP synthesis [39], and it lowers the ATP cost of contractile force production [40]. Thus, the greater SmO2RR observed in the present study suggests that beetroot consumption may have improved PCr resynthesis and, consequently, speed up muscle strength recovery. On the other hand, Siervo et al. (2016) [41] did not observe any significant effect on MVC after 7 days of beetroot juice consumption (~12 mmol/140mL). It is worth mentioning that the authors did not evaluate MVC in response to a high-intensity rhythmic handgrip exercise as has been done in the present study.
In summary, we investigated the effects of a single dose of a beetroot-based nutritional gel, rich in dietary nitrate, on SmO2, blood volume, and forearm muscle strength in older adults. The results of the present study suggest that the dietary nitrate improves SmO2 status of the forearm in older adults by increasing muscle O2 extraction during handgrip exercise and by speeding up muscle O2 resaturation during exercise recovery, thereby preventing the age-related prolongation in post-exercise recovery time of muscle O2 resaturation and improving the muscle function.
Supporting information
(PDF)
Values are expressed as means ± SD. BG = beetroot-based nutritional gel, PLA = nitrate-depleted gel, O2Hb = muscle oxyhaemoglobin, HHb = muscle deoxyhaemoglobin. * Significantly different from PLA. a significantly different from Pre.
(DOCX)
Values are expressed as means ± SD. BG = beetroot-based nutritional gel, PLA = nitrate-depleted gel, O2Hb = muscle oxyhaemoglobin. * Significantly different from PLA.
(DOCX)
Acknowledgments
The authors would like to thank Ricky Toledano for the preparation of the English version of the manuscript. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Research Foundation of the State of Rio de Janeiro – FAPERJ (E-26/110.309/2014). Funding was also provided from The Brazilian National Council for Scientific and Technological Development – CNPq (process no. 442977/2014-0). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120(9):357–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhambhani YN. Muscle oxygenation trends during dynamic exercise measured by near infrared spectroscopy. Can J Appl Physiol 2004;29(4):504–523. [DOI] [PubMed] [Google Scholar]
- 3.Kutsuzawa T, Shioya S, Kurita D, Haida M, Yamabayashi H. Effects of age on muscle energy metabolism and oxygenation in the forearm muscles. Med Sci Sports Exerc 2001;33(6):901–6. [DOI] [PubMed] [Google Scholar]
- 4.Ichimura S, Murase N, Osada T, Kime R, Homma T, Ueda C, et al. Age and activity status affect muscle reoxygenation time after maximal cycling exercise. Med Sci Sports Exerc 2006;38(7):1277–81. doi: 10.1249/01.mss.0000227312.08599.f1 [DOI] [PubMed] [Google Scholar]
- 5.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191(1):59–66. [DOI] [PubMed] [Google Scholar]
- 6.Baião D, Conte-Junior CA, Paschoalin VM, Alvares TS. Beetroot juice increase nitric oxide metabolites in both men and women regardless of body mass. Int J Food Sci Nutr 2016;67(1):40–6. doi: 10.3109/09637486.2015.1121469 [DOI] [PubMed] [Google Scholar]
- 7.Masschelein E, Van Thienen R, Wang X, Van Schepdael A, Thomis M, Hespel P. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J Appl Physiol 2012;113(5):736–45. doi: 10.1152/japplphysiol.01253.2011 [DOI] [PubMed] [Google Scholar]
- 8.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol 2009;107(4):1144–55. doi: 10.1152/japplphysiol.00722.2009 [DOI] [PubMed] [Google Scholar]
- 9.Breese BC, McNarry MA, Marwood S, Blackwell JR, Bailey SJ, Jones AM. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am J Physiol Regul Integr Comp Physiol 2013;305(12):R1441–50. doi: 10.1152/ajpregu.00295.2013 [DOI] [PubMed] [Google Scholar]
- 10.Aucouturier J, Boissière J, Pawlak-Chaouch M, Cuvelier G, Gamelin FX. Effect of dietary nitrate supplementation on tolerance to supramaximal intensity intermittent exercise. Nitric Oxide 2015;49:16–25. doi: 10.1016/j.niox.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 11.Oliveira GV, Morgado M, Pierucci AP, Alvares TS. A single dose of a beetroot-based nutritional gel improves endothelial function in the elderly with cardiovascular risk factors. J Funct Foods 2016;26:301. [Google Scholar]
- 12.Morgado M, Oliveira GV, Vasconcellos J, Monteiro ML, Conte-Junior C, Pierucci AP, et al. Development of a beetroot-based nutritional gel containing high content of bioaccessible dietary nitrate and antioxidants. Int J Food Sci Nutr 2016;67(2):153–60. doi: 10.3109/09637486.2016.1147531 [DOI] [PubMed] [Google Scholar]
- 13.Croitoru MD. Nitrite and nitrate can be accurately measured in samples of vegetal and animal origin using an HPLC-UV/VIS technique. J Chromatogr B Analyt Technol Biomed Life Sci 2012;911:154–161. doi: 10.1016/j.jchromb.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 14.Beer A. Versuch der absorptions-Verhältnisse des Cordierites für rothes Licht zu bestimmen. Ann Physik Chem 1851; 84: 37–52. [Google Scholar]
- 15.Van Beekvelt MC, Borghuis MS, van Engelen BG, Wevers RA, Colier WN. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin Sci (Lond) 2001;101(1):21–8. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari M, Wei Q, Carraresi L, De Blasi RA, Zaccanti G. Time-resolved spectroscopy of the human forearm. J Photochem Photobiol B 1992;16(2):141–53. [DOI] [PubMed] [Google Scholar]
- 17.Essenpreis M, Elwell CE, Cope M, van der Zee P, Arridge SR, Delpy DT. Spectral dependence of temporal point spread functions in human tissues. Appl Opt 1993;32(4):418–25. doi: 10.1364/AO.32.000418 [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S, Takasaki S, Ozaki T and Kobayashi Y. Tissue oxygenation monitor using NIR spatially resolved spectroscopy. Proc. SPIE 3597, Optical Tomography and Spectroscopy of Tissue III, 582 (July 15, 1999).
- 19.Patterson MS, Chance B, Wilson BC. Time resolved reflectance and transmittance for the non-invasive measurement of tissue optical properties. Appl Opt 1989;28(12):2331–6. doi: 10.1364/AO.28.002331 [DOI] [PubMed] [Google Scholar]
- 20.Beam WC, Adams GN (2014) Isometric (Static) Strength In: Exercise Physiology Laboratory Manual, 7th edn. New York: McGrall-Hill; 47–54. [Google Scholar]
- 21.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015;65(2):320–7. doi: 10.1161/HYPERTENSIONAHA.114.04675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bondonno CP, Liu AH, Croft KD, Ward NC, Shinde S, Moodley Y, et al. Absence of an effect of high nitrate intake from beetroot juice on blood pressure in treated hypertensive individuals: a randomized controlled trial. Am J Clin Nutr 2015;102(2):368–75. doi: 10.3945/ajcn.114.101188 [DOI] [PubMed] [Google Scholar]
- 23.Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol 2012;590(21):5361–70. doi: 10.1113/jphysiol.2012.239053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 2012;111(2):220–30. doi: 10.1161/CIRCRESAHA.112.269571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hearon CM Jr, Dinenno FA. Regulation of skeletal muscle blood flow during exercise in ageing humans. J Physiol 2016;594(8):2261–73. doi: 10.1113/JP270593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89 Pt B:122–35. doi: 10.1016/j.yjmcc.2015.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57(3):363–9. doi: 10.1161/HYPERTENSIONAHA.110.167015 [DOI] [PubMed] [Google Scholar]
- 28.Conley KE, Esselman PC, Jubrias SA, Cress ME, Inglin B, Mogadam C, et al. Ageing, muscle properties and maximal O2 uptake rate in humans. J Physiol 2000;526 Pt 1:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaughan RA, Gannon NP, Carriker CR. Nitrate-containing beetroot enhances myocyte metabolism and mitochondrial content. J Tradit Complement Med 2015;6(1):17–22. doi: 10.1016/j.jtcme.2014.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab 2011;13(2):149–59. doi: 10.1016/j.cmet.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 31.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA 2004;101(47):16507–12. doi: 10.1073/pnas.0405432101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci 2006;119(Pt 14):2855–62. doi: 10.1242/jcs.03062 [DOI] [PubMed] [Google Scholar]
- 33.Kime R, Hamaoka T, Sako T, Murakami M, Homma T, Katsumura T, Chance B. Delayed reoxygenation after maximal isometric handgrip exercise in high oxidative capacity muscle. Eur J Appl Physiol. 2003;89(1):34–41. doi: 10.1007/s00421-002-0757-3 [DOI] [PubMed] [Google Scholar]
- 34.Chance B, Dait MT, Zhang C, Hamaoka T, Hagerman F. Recovery from exercise-induced desaturation in the quadriceps muscles of elite competitive rowers. Am J Physiol 1992;262(3 Pt 1):C766–75. [DOI] [PubMed] [Google Scholar]
- 35.Boushel R, Pott F, Madsen P, Rådegran G, Nowak M, Quistorff B, et al. Muscle metabolism from near infrared spectroscopy during rhythmic handgrip in humans. Eur J Appl Physiol Occup Physiol 1998;79(1):41–8. doi: 10.1007/s004210050471 [DOI] [PubMed] [Google Scholar]
- 36.Hanada A, Okita K, Yonezawa K, Ohtsubo M, Kohya T, Murakami T, et al. Dissociation between muscle metabolism and oxygen kinetics during recovery from exercise in patients with chronic heart failure. Heart 2000;83(2):161–6. doi: 10.1136/heart.83.2.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brizendine JT, Ryan TE, Larson RD, McCully KK. Skeletal muscle metabolism in endurance athletes with near-infrared spectroscopy. Med Sci Sports Exerc 2013;45(5):869–75. doi: 10.1249/MSS.0b013e31827e0eb6 [DOI] [PubMed] [Google Scholar]
- 38.McCully KK, Iotti S, Kendrick K, Wang Z, Posner JD, Leigh J Jr, et al. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J Appl Physiol 1994;77(1):5–10. [DOI] [PubMed] [Google Scholar]
- 39.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol 2011;589(Pt 22):5517–28. doi: 10.1113/jphysiol.2011.216341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, et al. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol 2010;109(1):135–48. doi: 10.1152/japplphysiol.00046.2010 [DOI] [PubMed] [Google Scholar]
- 41.Siervo M, Oggioni C, Jakovljevic DG, Trenell M, Mathers JC, Houghton D, et al. Dietary nitrate does not affect physical activity outcomes in health older adults in a randomized, crossover trial. Nutr Res 2016;36(12): 1361–1369. doi: 10.1016/j.nutres.2016.11.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Values are expressed as means ± SD. BG = beetroot-based nutritional gel, PLA = nitrate-depleted gel, O2Hb = muscle oxyhaemoglobin, HHb = muscle deoxyhaemoglobin. * Significantly different from PLA. a significantly different from Pre.
(DOCX)
Values are expressed as means ± SD. BG = beetroot-based nutritional gel, PLA = nitrate-depleted gel, O2Hb = muscle oxyhaemoglobin. * Significantly different from PLA.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.