Table 1.
Optimization of aldol reactions using method Aa
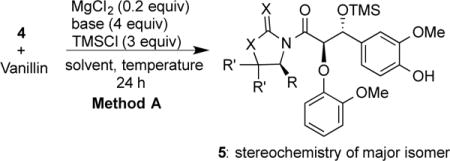
| ||||
|---|---|---|---|---|
|
| ||||
| entry | compd | base | solvent | diastereoselectivityb |
| 1 | 4a | Et3N | CH2Cl2 | 78:7:6:9 (rt) |
| 2 | 4a | Et3N | CH2Cl2 | 80:12:4:4 |
| 3 | 4c | Et3N | CH2Cl2 | 78:4:16:2 (rt) |
| 4 | 4e | Et3N | CH2Cl2 | 72:9:10:9 (rt) |
| 5 | 4f | Et3N | CH2Cl2 | 57:27:16 (rt)c |
| 6 | 4g | Et3N | CH2Cl2 | 31:16:53 (rt)c |
| 7 | 4a | BuNH2 | CH2Cl2 | trace product |
| 8 | 4a | PhNH2 | CH2Cl2 | no product |
| 9 | 4a | i-Pr2NH | CH2Cl2 | 73:15:4:8 |
| 10 | 4a | DIPEAd | CH2Cl2 | no product |
| 11 | 4a | pyridine | CH2Cl2 | no product |
| 12 | 4a | DBUe | CH2Cl2 | no product |
| 13 | 4a | Et3N | ClCH2CH2Cl | 72:16:7:5 |
| 14 | 4a | Et3N | EtOAc | 76:7:8:9 |
| 15 | 4a | Et3N | THF | 71:2:7:20 |
| 16 | 4a | Et3N | Et2O | 64:6:14:16 |
| 17 | 4a | Et3N | toluene | 57:6:6:31 |
| 18 | 4a | Et3N | CH3CN | no product |
Unless indicated, all reactions were carried out at 0 °C;
Diastereoselectivity was determined by HPLC. See SI for details;
Diastereomers were inseparable under this HPLC condition;
Diisopropylethylamine;
1,8-Diazabicyclo[5.4.0]undec-7-ene.
