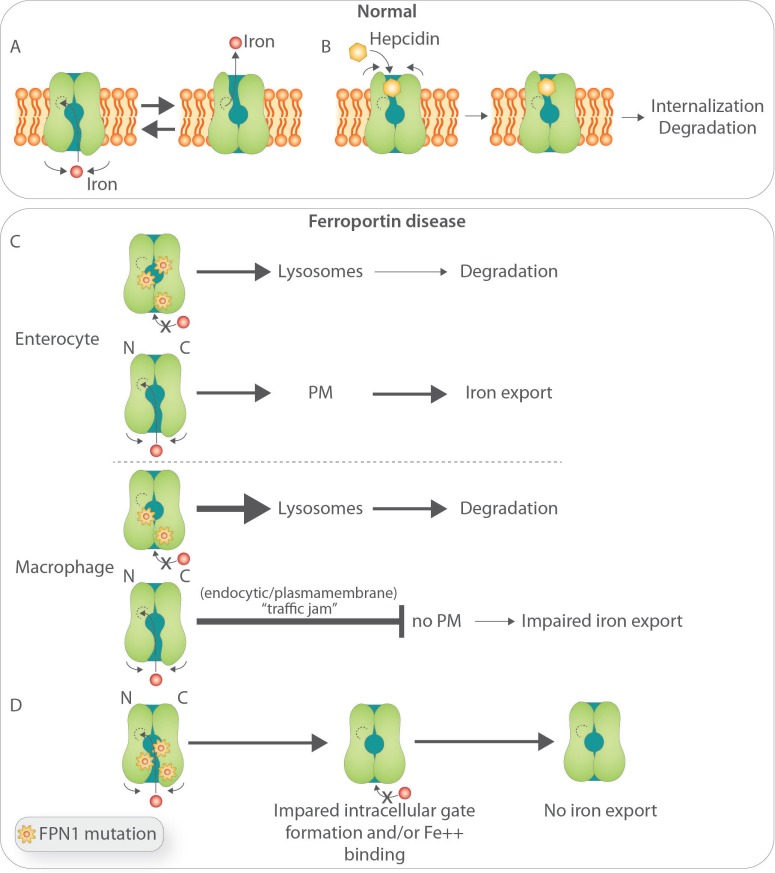
Figure 1.
Biology of ferroportin and postulated pathobiology of Ferroportin Disease (FD). (A) Structure-function relationship of iron-export ferroportin activity.39 (B) Putative mechanisms of hepcidin binding to FPN and its degradation.39 (C) Postulated basis for FD. (Upper panel) In cells undergoing relatively low iron flux, such as enterocytes, the product of the FPN wild-type allele is able to reach the plasma membrane and export iron. For clarity, mutated FPN1 was not depicted at the cell surface: based on previous in vitro work, it has been postulated that some mutant FPN1 can still reach the cell surface and preserve some iron-transport competence, but this is still controversial. (Lower panel) In cells undergoing high iron turnover, such as macrophages, increased requests for iron export impose high demands on FPN traffic leading to a ‘traffic jam’ within the endocytic/plasmamembrane and degradation compartments and inappropriately low wild-type allele product targeting to the cell membrane.54 (D) Postulated effect of FPN mutations that affect formation of the intracellular gate and access to the iron binding site.39