Figure 1.
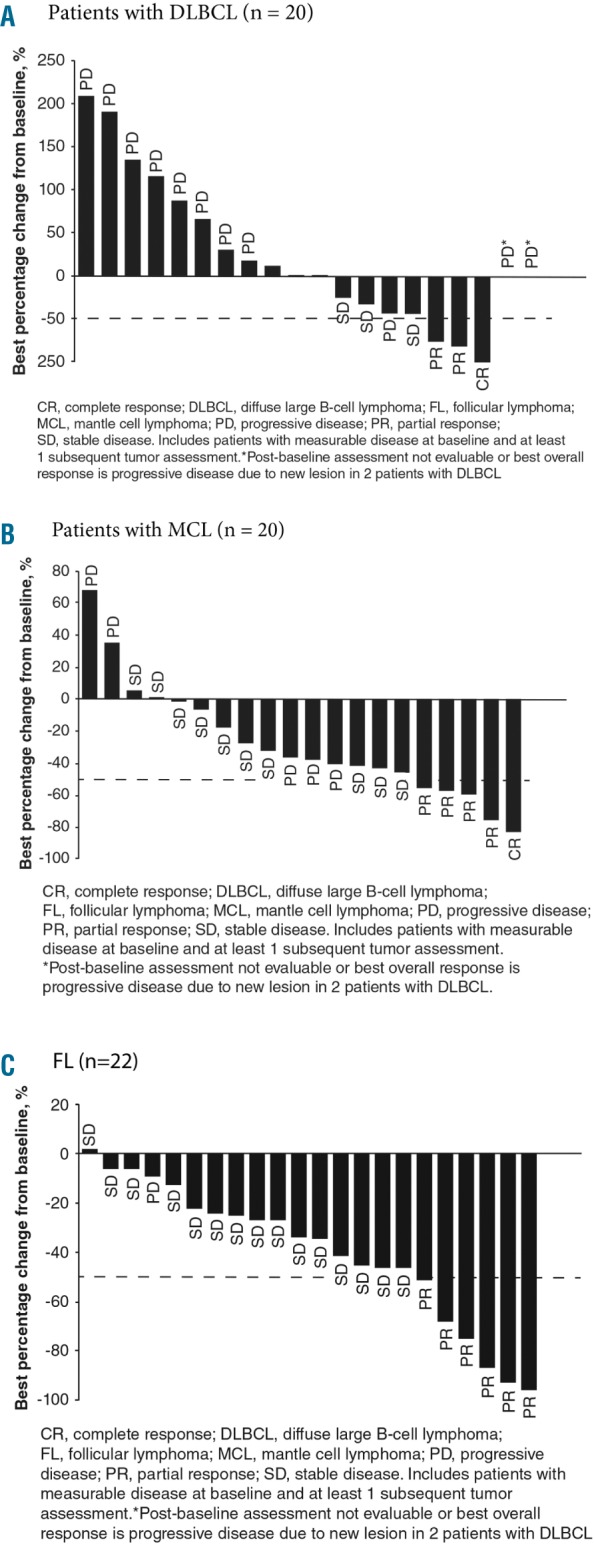
Best overall response with respect to best percentage change from baseline in investigator-assessed tumor size during buparlisib therapy for patients with measurable lesions divided by cohort The graphs include patients with measurable disease at baseline and ≥1 subsequent tumor assessments. The data cut-off was February 25, 2015, for all cohorts. (A) Patients with DLBCL (n=20), (B) patients with MCL (n=20), (C) patients with FL (n=22). Best overall response is indicated for each patient. Note that for two patients with DLBCL, the post-baseline assessment was not evaluable or best overall response was progressive disease due to a new lesion (asterisk). The dashed line shows the percentage change that represents the criterion for response, according to International Working Group criteria.34,35 CR: complete response; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; IWG: International Working Group; MCL: mantle cell lymphoma; PD: progressive disease; PR: partial response; SD, stable disease.
