Abstract
Patient engagement through shared decision-making (SDM) is increasingly seen as a key component for patient safety, patient satisfaction, and quality of care. Current SDM models do not adequately account for medical and environmental contexts, which may influence medical decisions in the hospital. We identified leading SDM models and reviews to inductively construct a novel SDM model appropriate for the inpatient setting. A team of medicine and pediatric hospitalists reviewed the literature to integrate core SDM concepts and processes and iteratively constructed a synthesized draft model. We then solicited broad SDM expert feedback on the draft model for validation and further refinement. The SDM 3 Circle Model identifies 3 core categories of variables that dynamically interact within an “environmental frame.” The resulting Venn diagram includes overlapping circles for 1)patient/family, 2)provider/team, and 3)medical context. The environmental frame includes all external, contextual factors that may influence any of the 3 circles. Existing multistep SDM process models were then re-articulated and contextualized to illustrate how a shared decision might be made. The SDM 3 Circle Model accounts for important environmental and contextual characteristics that vary across settings. The visual emphasis generated by each “circle” and by the environmental frame direct attention to often overlooked interactive forces and has the potential to more precisely define, promote, and improve SDM. This model provides a framework to develop interventions to improve quality and patient safety through SDM and patient engagement for hospitalists.
Keywords: Shared decision-making, medical decision-making, patient engagement, patient-centered care, physician-patient communication
Introduction
Evolving models of medical care emphasize the importance of shared decision-making (SDM) on practical and ethical grounds.1–3 SDM is a cognitive, emotional, and relational process where provider and patient collaborate in a decision after discussing the options, evidence, and potential benefits and harms, while considering the patient’s values, preferences, and circumstances.4 Categories of decisions include information gathering, pharmacotherapy, therapeutic procedures, consultations and referrals, counseling and precautions (e.g., behavior modification, goals of care, end-of-life care), and care transitions (e.g., transfer or discharge to home).5 Decisions span the continuum of urgency and may be anticipatory or reactive.6 The patient’s environment7,8 and the provider-patient relationship9 have been explicitly incorporated into the ideal SDM process.
SDM has been conceptually and empirically linked with evidence-based practice,1 although the relationship between SDM and clinical outcomes is less clear.10,11 SDM is desired by patients12 and may bolster patient satisfaction, trust, and adherence.13,14 Limited evidence suggests SDM could reduce inappropriate treatments and testing,15 decrease adverse events,16 and promote greater patient safety,17–19 but more well-designed studies are needed.
Provider, patient, and contextual factors influence the extent to which SDM occurs. Providers commonly cite time constraints and perceived lack of applicability to certain clinical scenarios or settings,19 Providers may also lack training and competency in SDM skills.2 Patients may be reluctant to disagree with their provider or fear being mislabeled as “difficult.”20 When faced with high stakes or emotionally-charged decisions, patients’ surrogates may prefer to have the provider serve as the sole decision-maker.21 Contextually, there may be limited evidence, high clinical stake, or a number of equally beneficial (or harmful) options.22,23
Current SDM models guide clinicians in determining when and how to engage in SDM, yet models vary widely. For example, Elwyn’s model emphasizes the ethical imperative for SDM and outlines 3 SDM steps: introduce choice, describe options, and help patients explore preferences and make decisions.3 Using a multi-modal review and clinician-driven feedback, Legaré’s “IP-SDM” model illustrates the roles of the interprofessional team and emphasizes the influence of environmental factors on decision-making.24 Recent systematic reviews of SDM models have attempted to identify common elements, language, and processes.2,25,26
While published SDM models demonstrate varying emphases – e.g., evidence-based medicine,2 provider-patient relationships,9 interprofessional practices and environmental influences,24 or patient contextual factors 7,8 – none specifically addresses hospitalization and the issues that impact decisions as a patients’ clinical condition and care needs change. Studies of SDM in hospitalized patients have relied on either general theoretical frameworks for patient engagement, or on conceptual models developed specifically for outpatient care.16,27,28 While the key tenets of SDM are relevant across clinical settings, hospitalization introduces a number of unique and highly relevant factors that may influence all aspects of the SDM process. Table 1 provides several examples from the authors of how inpatient and outpatient SDM may differ.
Table 1.
Examples of differences between inpatient and outpatient shared decision-making
| Outpatient Setting | Inpatient Setting |
|---|---|
Timing / Temporality of Decisions
|
Timing / Temporality of Decision
|
Decision-making Environment
|
Decision-making Environment
|
Disease Acuity
|
Disease Acuity
|
Relationships between decision makers
|
Relationships between decision makers
|
Common Issues
|
Common Issues
|
This study reviews leading SDM models to construct a more environmentally and contextually sensitive model appropriate for the hospital setting. While developed with hospital medicine in mind, a synthesized model that attends to environmental and systems context, provider/team factors, patient factors, and disease/medical variables is highly relevant in any setting where SDM occurs.
Methods
We constructed a model appropriate for SDM across the care continuum through the following 3-part, iterative group process: 1) a comprehensive literature review of existing SDM models, 2) synthesis and inductive development of a new draft model, and 3) modification of the new model using feedback from SDM experts.
Narrative literature review
We performed a structured, comprehensive literature review 29 to compare and contrast existing SDM models and frameworks. Leading models and key concepts were first identified using 2 systematic reviews 25,26 and a comprehensive review.2 In order to extend the search to 2016 and include any overlooked articles, a PubMed search was performed using the terms “shared decision-making” or “medical decision-making” AND “model” or “theory” or “framework” for English-language articles from inception to 2016. The search was repeated using Google Scholar to verify results and obtain the number of citations per article as a proxy for impact and saturation. In order to minimize possible search error or selection bias, reference lists in high impact publications were hand searched to identify additional articles. All abstracts were manually reviewed by 2 independent authors for relevance and later inclusion in our group iterative process. A priori inclusion criteria were limited to provider-patient SDM (i.e. not clinical reasoning or making decisions in general) and complete descriptions of a conceptual model or framework. Additional publications suggested by experts (e.g., perspective pieces or terminology summaries) were also reviewed.
Model development and expert review
An electronic SDM reference library and annotated bibliography of the selected articles (Table 2) was created to guide the synthesis of SDM models and highlight needed revisions for hospital medicine. In a process similar to Legaré,24 a group of 8 pediatric and adult medicine hospitalists, a palliative care physician, a cognitive psychologist, a biostatistician, and 3 medical trainees reviewed the selected SDM publications and models30 and independently created their own adapted inpatient SDM models. Through an iterative, consensus-building group process, each model was discussed to select key elements or features to be integrated into a synthesized model. This model was guided by principles of social ecological theory, which emphasizes the role of the individual as influenced by and interactive with systems and the environment.31
Table 2.
Annotated list of selected SDM studies and models/frameworks
| Author(s) and Citation | Description |
|---|---|
| Braddock CH, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. 199957 | Using a cross-sectional descriptive evaluation of audiotaped office visits of primary care and surgeon office visits. Informed (shared) decision-making was found to be incomplete. Conclusion: More needs to be done to encourage SDM. |
| Braddock III CH, Fihn SD, Levinson W. et al. 199756 | Cross-sectional descriptive evaluation of informed decision-making based on audiotaped primary care office encounters. Authors used six criteria to score informed decision-making and found that a discussion of risks and benefits and patient understanding was infrequent. |
|
Charles C, Gafni A, Whelan T. 199751 Charles C, Gafni A, Whelan T. 19994 |
Landmark studies that described a framework for shared decision-making based on a physician-patient partnership in the decision-making process. The process included sharing of information including treatment preferences and agreement on a decision. |
| Elwyn G, Frosch D, Thomson R, et al. 20123 | Authors describe an SDM model for treatment decision in primary care. The model focuses on patient’s active involvement in the process, exploration of expectations and options, teach back and follow up. Three key steps include choice talk, option talk and decision talk. |
| Elwyn G, Lloyd A, May C, et al. 201437 | Authors describe the collaborative deliberation model of decision-making based on five communicative efforts of constructive interpersonal engagement, recognition of alternative actions, comparative learning, preference construction and elicitation and preference integration. The model could apply to different types of communication in health care including motivational interviewing, SDM, goal setting and action planning. |
| Epstein RM, Gramling RE. 201353 | Review of the SDM in the context of complex and uncertain situations and the role of preference, relationship and the concept of shared attentional focus. Authors also include the role of information technology, health care teams and health systems in decision-making. |
| Hoffmann TC, Montori VM, Del Mar C. 20141 | Authors highlight the interconnection between evidence-based medicine (EBM) and SDM - each is necessary in combination to improve patient care. Calls for SDM and EBM to be included in practice guidelines and future research. |
| Holzmueller CG, Wu AW, Pronovost PJ. 201236 | Framework for physicians to determine patient involvement in decision-making and includes patient-related factors. The framework further delineates situations when patients should decide and when physicians should decide. |
| Kon, AA. 201054 | Commentary describes SDM as a continuum with one end being patient driven and the opposite physician driven with a middle being both as equal partners. Different decisions and situations call for varying degrees of patient and physician input in the process. |
|
Légaré F, Stacey D, Pouliot S, et al. 201140 Légaré F., Stacey D, Gagnon S. 201160 |
The model describes an interprofessional approach to SDM. Each professional works either in collaboration with other providers or sequentially with the patient. The model includes the role of environment in SDM and includes clarification of values and feasibility of options. |
| Makoul G, Clayman ML. 200625 | Literature review of SDM models and propose a model based on 9 essential elements. The elements include: define/explain problem, present options, discuss pros/cons, patient preferences/values, patient ability, physician recommendations, checking for understandings, make/defer decision and arrange follow up. Authors also include ideal elements and general qualities that promote SDM. |
| Moumjid N, Gafni A, Bremond A, et al. 200726 | Explores if there is a clear definition of SDM, whether authors provide a definition of SDM when they use the term, and whether they are consistent in doing so. |
| Muëller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. 201145 | This paper investigates current social norms regarding the appropriateness of SDM in different situations. The authors find that SDM is considered most important in severe illness and chronic condition. SDM was also indicated as necessary when there is more than one therapeutic option without one being clearly superior. |
| Rapley T. 200855 | Describes a framework for how to conceptualize decision-making as an evolving series of encounters over time interfacing with several different individuals, knowledge acquisitions and technologies. |
| Stacey D, Légaré F, Pouliot S, et al. 201052 | Comprehensive theory analysis of SDM conceptual models to determine how relevant they are to interprofessional collaboration in clinical practice. They concluded that most SDM models did not utilize an interprofessional approach. This highlights the need for a model that is more inclusive of other health professionals. |
| Torke AM, Petronio S, Sachs GA, et al. 201234 | This article uses literature from medicine, communication studies, and medical ethics to build a conceptual model of the role of communication in decision-making. Information processing and relationship building were found to be two major elements of interpersonal communication. |
| Towle A, Godolphin W. 199959 | Model is developed from proposed physician and patient competencies for learning and teaching SDM. The competencies include developing a physician-patient partnership, explicit discussion around patient preference and readiness, role of the patient in the decision-making process, developing an action plan and resolving conflict. |
| Weiner SJ, Schwartz A, Sharma G, et al. 20138 | Observational study using a protocol of medical chart audits and audiotaped provider encounters at internal medicine clinics at 2 VA hospitals to evaluate for contextualizing care (also called patient-centered decision-making); providers were scored on their ability to incorporate contextual factors such as barriers to treatment into care planning. The developed protocol could be used to assess physician performance around contextualized decision-making. |
| Whitney SN. 200323 | This article proposes a model of medical decisions based on importance and clarity. It also identifies 3 types of decisions that are less well suited to a collaborative decision: major decisions with low certainty, minor decisions that have high certainty, and major decisions that have high certainty when patients and physicians disagree. |
The draft model and a standardized set of questions (Appendix A) were then emailed to all first and last authors of the reviewed studies (Table 2). Expert responses were compiled, coded, and analyzed independently by 3 co-authors. Inductive coding techniques and a constant comparative approach were used to code the qualitative data.32 Preliminary findings were shared among the 3 reviewers and discussed until consensus was reached on emerging themes and implications for the new SDM model and multistep SDM pathway. A master list of suggested revisions was shared with the larger authorship team and the model was refined accordingly.
Results
Two previously published systematic reviews25,26 identified 494 articles, 161 conceptual definitions of SDM, and over 30 separate key concepts. The additional PubMed search garnered 1,957 publications (with many overlapping from the systematic reviews). A manual search of the systematic reviews and PubMed abstracts identified 16 unique and complete decision-making models for further review. Hand searches of their citations yielded an additional 6 models for a total of 22 models.3,4,13,23,33–51 The majority of excluded articles described specific decision aids and small clinical studies, focused on only one step of the decision-making process, or were not otherwise relevant. The first (SR) and senior authors (JS) reviewed the 22 models for SDM relevance, generalizability, and content saturation yielding a final sample of 9 SDM models. A subsequent Google Scholar search did not identify any new SDM models but 2 SDM theory papers52,1 and 2 commentaries53,54 were selected based on influence (i.e. number of citations), expert recommendation or coverage of a novel aspect of SDM. A total of 15 studies (9 SDM models + 6 reviews; See Table 2) were used by our development team to create a synthesized SDM model. A tenth SDM model55 and 3 additional descriptive and normative studies8,56,57 were later added based on expert feedback and incorporated into our final SDM 3 Circle Model.
Expert Feedback
Twenty-one out of 27 (78%) SDM expert authors responded to our email request for feedback. The majority (62%) agreed with the basic elements of the model, including the environmental frame and the 3 domains. Some respondents viewed SDM as strictly a process between patient and provider independent of the disease, leading to refinement of the medical context category. Several experts emphasized the importance of SDM “set-up,” which includes the elicitation of patient preferences in how decisions are made and the extent of patient and/or surrogate involvement.
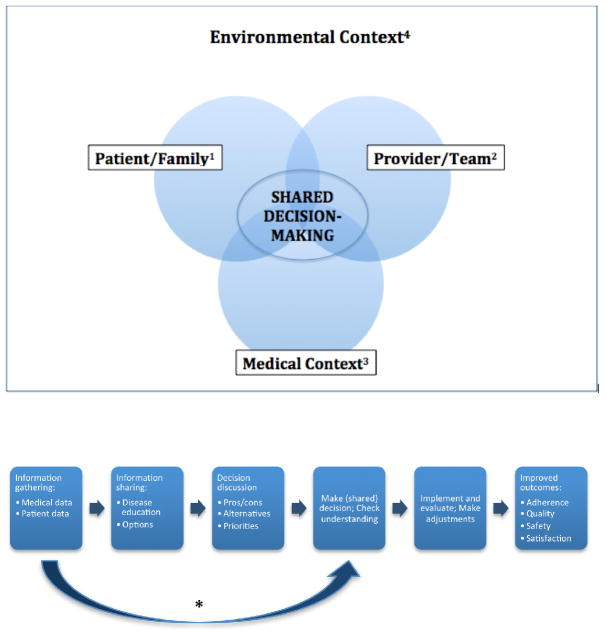
Several respondents identified time constraints (N=2), acuity of disease (N=3), and presence of multiple teams (N=6) to be the significant factors distinguishing inpatient from outpatient SDM. For some experts, “team” referred to the interprofessional care team while others referred to it as the collaboration among attending physicians and trainees. Experts noted that while the intensity and frequency of inpatient interactions could promote SDM, higher patient acuity and the urgency of decisions could negatively influence SDM and/or the patient’s ability to participate. Similarly, the presence of other team members may either impede or promote SDM by either contributing to miscommunication or bringing well-trained SDM experts to the bedside. Financial impact on patients and resource constraints were also noted as relevant. All of these elements have been incorporated into the final SDM 3 Circle Model and multistep SDM Pathway (Figure 1).
Figure 1. SDM 3-Circle Conceptual Model and Multistep Shared Decision-Making (SDM) Pathway.
1Patient/Family: A patient’s ability to engage in SDM reflects one’s health (e.g., functional and cognitive status) and life circumstances (e.g., socio-economic status; presence of a family member to serve as a surrogate).
2Provider/Team: SDM engagement is influenced by characteristics of an inpatient team (e.g., attending physician, trainees, nurse, social workers, case managers, dietitians, therapists) and characteristics of the healthcare providers it comprises (e.g., fatigued vs. well-rested; variable familiarity with SDM guidelines).
3Medical Context: Some decisions require a patient to provide informed consent (e.g., invasive hospital tests and procedures; blood product transfusions); others require a patient to play a fundamental role (e.g., adhere to prescription or course of rehabilitation).
4Environment: A clinical service (e.g., medicine or pediatrics, emergency department, hospital floor or intensive care unit) operates within a hospital (e.g., university-based/community-based) located in a community (e.g., transportation options) and health system (with varying incentives and priorities). Features of each level can influence the SDM encounter through their bearing on the three domains.
* Certain situations may warrant bypassing or limiting the steps of information sharing and decision discussion such as time-sensitive emergencies (e.g. emergency surgery) or if the patient and/or surrogate are uninterested or unable to participate in SDM
The SDM 3 Circle Model
The SDM 3 Circle Model comprises 3 categories of SDM barriers and facilitators that intersect within the environmental frame of an inpatient ward or other setting: 1) Provider/Team, 2) Patient/Family, and 3) Medical Context. A Venn diagram visually represents the conceptual overlaps and distinctions among these categories that are all affected by the environment in which they occur (Figure 1).
The patient/family circle mirrors prior SDM models that address the role of patient preferences in making decisions,3,4,12 with the explicit addition of the roles of families and surrogates as either decision makers or influencers. This circle includes personal characteristics, such as cognitions (e.g., beliefs, attitudes), emotions (e.g., anxiety, hope), behaviors (e.g., adherence, assertiveness), illness history (i.e. subjective experience and understanding of one’s own medical history), and related social features (e.g., culture, education, literacy, social supports).
Patient factors are not static over time or context. They occur within an environmental setting and are likely to be influenced by concurrent provider and medical variables (the second and third circles). Disease exacerbation leading to hospitalization or transfer to a subacute facility could dramatically shift the calculus a patient uses to determine preferences or activate dormant family dynamics. Strong provider-patient rapport (the overlap of patient and provider factors) may influence the development of trust and subsequent decisions.9 The type of disease or symptom presentation (circle 3 - medical context) may further influence patient factors due to stigma, perceived vulnerability, or assumed prognosis.
The provider/team circle includes both individual and team-based factors falling into similar categories as the patient/family domain such as cognitions, behavior, and social features; however, these factors include both personal (e.g., the provider’s personal history, values and beliefs) and professional (e.g., past medical training, decision-making style, past experiences treating a disease) characteristics. Decisions may involve an interprofessional team representing a broad range of personalities and professional values. Decisions and decision-making processes may change over time as team composition changes, as level of provider expertise varies, or as environmental, patient, or disease/illness factors influence providers and teams.
Medical context includes factors related to the disease and the potential ways to evaluate or manage it. Examples of disease factors include acuity, symptoms, course, and prognosis. Most obviously, disease factors will influence the content of risk-benefit discussions but may also affect the SDM process through disease stigma or cultural assumptions about etiology. Disease evaluation factors include the psychometrics of a diagnostic screen, invasive and non-invasive testing, or a range of different preventive or therapeutic interventions. Treatment variables include the available options, costs, and risk of complications. Medical context variables evolve as evidence-based medicine and biomedical knowledge increase and new treatment options emerge.
Each of the 3 circles operates within the same environmental frame, such as an inpatient medicine ward, which itself operates within a hospital and the broader health care system. This frame exerts overt and subtle influences on providers, patients, and even the medical context. Features of the environmental frame include culture (e.g. values, preferences, social norms), university vs. community setting, incentives, formularies, quality improvement campaigns, regulations, and technology use.
The dynamic interactivity of the environmental frame and the 3 circles inform the process of SDM and highlight key differences that may occur between care settings. Certain features may predominate in different situations, but all will influence and be influenced by features of other circles during the course of SDM.
Application of the SDM 3 Circle Model
As shown in Figure 1, the multistep SDM pathway begins with information gathering and processing where the provider solicits medical history as well as patient preferences for decision-making. This “processing” of patient decision-making preferences is less commonly described. The next steps, sharing information and decision discussion, include patient education about the medical issue and available treatments. Discussions may involve the pros/cons of each option, alternative diagnostic or management strategies, and how these decisions fit with a patient’s preferences, abilities (e.g., health literacy)58 and resources, or what has been called “contextualizing care.”7,8 Framing and other provider behaviors, including the use of decision aids and decision guides,15 may influence these conversations. Finally, after gathering, sharing, and discussing information (as influenced by the environment and 3 circles), a medical decision is made and patient understanding is verified. Detailed examples of how this model might be applied are illustrated with case scenarios in Appendix B.
While the SDM process is similar across clinical settings, its operationalization varies in important ways for hospital decision-making. In some situations, patients may defer all decisions to their providers or decisions may be considered with multiple providers concurrently. In the hospital SDM may not be possible such as in emergency surgery for an obtunded patient or patient and surrogate are not available or able to participate in the decision. Therefore, providers may bypass the steps of information sharing and discussion of the decision (big arrow in Figure 1 and Appendix B), proceeding directly to decision-making.
Discussion
The SDM 3 Circle Model provides a concise, ecologically valid, contextually sensitive representation of SDM that synthesizes and extends beyond recent SDM models.3,7,40 Each circle represents the forces that influence SDM across settings. While the multistep SDM pathway occurs similarly in outpatient and inpatient settings, how each step is operationalized and how each “circle” exerts its influence may differ and warrants further consideration throughout the SDM process. For example, hospitalized patients may have greater stress and anxiety, have more family involvement, be more motivated to adhere to treatment, and may be under greater financial and social pressures. Unlike outpatient primary care, patients are less likely to have an existing relationship with their inpatient providers, potentially compromising patient confidence in the provider, and necessitating expeditious trust building.
The SDM 3 Circle Model captures “setting” in both the broader environmental frame and within the provider/team category of variables. The frame also captures health system and broader community variables that may influence the practicality of some medical decisions. Within this essential frame, all 3 categories of patient, provider, and medical context are included as part of the SDM process. A better understanding of their interplay may be of great value for clinicians, researchers, administrators, and policy makers who wish to further study and promote SDM. Both the SDM 3 Circle Model and its accompanying pathway (Figure 1) highlight opportunities for intervention and research, and may drive quality improvement initiatives to improve clinical outcomes.
Limitations
We did not perform a new systematic review, potentially omitting lesser known publications. We mitigated this risk by using recent systematic reviews, searching multiple databases, hand searching citation lists, and making inquiries to SDM experts. Our selection of models used as a foundation for the synthesized model was based on consensus, which included an element of subjective, clinical judgment. Our SDM expert sample was small and limited to authors of the papers we reviewed, potentially restricting the range of viewpoints received. Lastly, the SDM 3 Circle Model highlights key concept areas rather than all possible factors that influence SDM.
Conclusion
We present a peer-reviewed, literature-based SDM model capable of accounting for the unique circumstances and challenges of SDM in the hospital. The SDM 3 Circle Model identifies the primary categories of variables thought to influence SDM, places them in a shared environmental frame, and visually represents their interactive nature. A multistep representation of the SDM process further illustrates how the unique features and challenges of hospitalization might exert influence at various points as patients and providers reach a shared decision. As the interrelationships of patient, provider/team, medical context and the environmental frame in which they occur are better understood, more effective and targeted interventions to promote SDM can be developed and evaluated.
Acknowledgments
Financial Support: This work was supported in part by funding from the National Institutes of Health. Funded by NIH (OBSSR/NCCIH) grant #R25006573 awarded to Dr. Jason Satterfield.
We would like to thank the authors for their feedback on the conceptual model, Evans Whitaker for his assistance with the literature review, and the Patient Engagement Project volunteers for their support and assistance with data collection.
Appendix A. Email template to SDM experts with initial SDM model and stimulus questions

I am writing to you based on your expertise in patient safety and patient communication to get your input on an SDM model. We have greatly benefited from your work in this area and have noticed that most of the SDM models focus on outpatient medicine. We have synthesized the best of those models into the attached, two-part figure that we believe can work for either outpatient or inpatient medicine and plan to publish. We would greatly appreciate it if you could look at this figure and give us your impressions. We have added a few prompts below but any comments or impressions are welcome. To date, our patients and providers have been extremely positive about our work and we hope to share our materials with others very soon.
Questions:
Do the 3 primary circles accurately capture the primary domains for SDM consideration? If not, what is missing?
Does the larger environmental context “frame” help? Our intent was to capture the environmental factors that might be different in an inpatient vs. outpatient setting.
At present, the SDM process pathway is essentially the same as outpatient medicine. What changes would you make to better represent the SDM process for inpatient medicine? (For example, we considered including the influence of other team members, location, and conversations with outpatient doctors, or the influence of acuity or co-morbidities.) Any suggestions?
Please add any general comments including those about uniqueness, clarity, visual appeal, etc.
Appendix B. Clinical application of the SDM Conceptual Model and Multistep Shared Decision-Making (SDM) Pathway
|
Case An 89-year-old woman with osteoporosis and Alzheimer’s dementia is hospitalized after a mechanical fall at home resulting in several rib fractures. Her hospitalization has been complicated by pain and delirium. Decision Type – Disposition/Care Transition: Discharge home or discharge to a skilled nursing facility Environmental Framework – Rural community hospital with limited choices for skilled nursing facilities in the area. Traditional physician-centric decision models are typically followed. There is pressure for quick discharge. Patient/Family – Patient has consistently expressed a wish to return home but has been delirious in the hospital. This is her second hospitalization for falls. The patient’s spouse died several years ago in a long-term care facility. The patient and her family had a negative experience and believe that the facility will not provide enough supervision and attention to her. Provider/Team – The hospitalist is concerned that the patient’s delirium may not improve quickly in the skilled nursing facility but home would not provide regular physical therapy, occupational therapy or nursing care. The team, including the hospitalist, bedside nurse, physical therapist, occupational therapist and case manager recently discharged another patient home in the past week who was discharged home and was readmitted less than 48 hours later with a hip fracture after a fall. Medical context –The patient is medically stable for discharge. The closest nursing facility is 65 miles from the patient’s home but the patient would receive at least one hour of physical therapy every day. The patient’s niece and primary caregiver do not have a car and would have to travel by bus to visit the patient. If the patient is discharged home she would receive home physical therapy twice a week. |
|
|
| Case 2 |
| A 13-month-old fully immunized girl with a history of febrile seizures is admitted with fevers and lethargy. She is diagnosed with Streptococcus pneumoniae meningitis and the inpatient team’s recommendation is a minimum 10-day course of parenteral antibiotic treatment. Decision Type – Treatment: Placing an intravenous (IV) access for prolonged parenteral therapy and includes the options of long term access with antibiotics at home versus short term access while remaining in the hospital for the duration of therapy Environmental Framework – Admitted to a children’s hospital with resources and availability of several types of procedures for short term and long term IV access. Availability of resources such as home care and home pharmacy may factor into whether or not it could be an option for the family to administer the antibiotic therapy at home. Patient/Family – There is no history or experience with procedures and inpatient hospitalization. There is support at home and the ability of family to care for the child. Provider/Team – The hospitalist’s bias includes a personal preference for long term access. Medical Context – The decision is based on evidence-based guidelines and there is one option that the hospitalist is recommending. An oral course of antibiotic or shorter duration of treatment should not be posed as an option to the family since this practice departs from the standard of care. However, the route by which she receives the intravenous (IV) antibiotic could be a discussion that engages the family’s preferences and values. Although peripheral IVs are less invasive, she would require multiple IVs through the course of treatment since most IVs last for only 2–3 days. Because of her young age and smaller veins, IV access may become more challenging as she continues therapy and she may require several attempts before IV access is secured. However, placement of a PICC line carries the risks associated with a central line such as bloodstream infection and blood clots. Another consideration with this particular age group is that she would likely need to undergo moderate sedation for placement of the PICC line, which carries its own inherent risks. If she does undergo PICC line placement however, the family may also have the option of administering the antibiotic therapy at home. |

| SDM Pathway Step and Application (see Figure 1) | Case 1 | Case 2 |
|---|---|---|
| Information gathering | Where would you like to go after hospital discharge? What kind of support services are needed if you were to go home? What experience have you had with nursing facilities? | How has she been tolerating peripheral access this hospitalization? Who is available to place central lines here? What antibiotic is recommended? What is the duration of therapy? |
| Information sharing | During your hospital stay these events happened… There are the options available after you leave the hospital… We anticipate that…when you leave the hospital. |
Bacterial meningitis is… Based on current evidence-based guidelines antibiotic therapy is… These are the options for how your daughter may receive the antibiotic therapy… |
| Decision discussion | As we discuss your recovery what is important to you in terms of care? Let’s compare what your recovery would be like at home versus a nursing facility. |
The benefits of using a PICC line include … and the risks include… If she receives a PICC line and does therapy at home …if she stays in the hospital… What do you think would work best for her? Tell me more about that. |
| SDM | The decision is to go home. Tell me what factored into our decision for her to go home and what we discussed may happen at home. | We have made the decision to go ahead and place a PICC line and receive antibiotics at home. Based on what we discussed tell me what will happen when you arrive at home. |
| Implementation | Home nursing services and physicial therapy has been set up. Additionally the team has recommended home occupational therapy. | The PICC has been placed. Our team wants to help with the transition home and have arranged for bloodwork to be drawn in one week at the Pediatrician’s clinic. What questions do you have? |
| Outcomes | A visiting nurse will follow up on pain and functional status during the home visits. The nurse will continue to communicate with the patient, caregivers, hospital providers and the primary care team on medication adherence and falls prevention. | Following discharge the pediatric nurse will call the family twice a week to answer any questions and ask about treatment response, side effects and follow up care. |
Footnotes
Financial support for this study was provided entirely by a grant from NIH/NCCIH (grant #R25 AT006573). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The following authors are employed by the sponsor: Stephanie Rennke, M.D., Patrick Yuan, B.A., Brad Monash, M.D., Rebecca Blankenburg, M.D., M.P.H., Ian Chua, M.D., Stephanie Harman, M.D., Debbie S. Sakai, M.D., Joan F. Hilton, D.Sc., M.P.H., and Jason Satterfield, Ph.D.
Author Disclosure Statement: The authors do not have any disclosures to report.
Contributor Information
Stephanie Rennke, Department of Medicine, University of California, San Francisco, 533 Parnassus Avenue, U101A, San Francisco, CA 94143, USA.
Patrick Yuan, Department of Medicine, University of California, San Francisco, 1545 Divisadero Street, Suite 322, Box 0320, San Francisco, CA 94115, USA.
Brad Monash, Department of Medicine, University of California, San Francisco, 505 Parnassus Avenue, Box 0131, San Francisco, CA 94143, USA.
Rebecca Blankenburg, Department of Pediatrics, Lucile Packard Children’s Hospital, Stanford University, 725 Welch Road, Palo Alto, CA 94304, USA.
Ian Chua, Division of Hospital Medicine, Department of Pediatrics, Children’s National Medical Center, George Washington School of Medicine, 111 Michigan Avenue NW, Washington, DC 20010, USA.
Stephanie Harman, Department of Medicine, Stanford University, 1265 Welch Road, Stanford, CA 94305, USA.
Debbie S. Sakai, Department of Pediatrics, Lucile Packard Children’s Hospital, Stanford University, 725 Welch Road, Palo Alto, CA 94304, USA.
Adeena Khan, Department of Medicine, University of California, San Francisco, 533 Parnassus Avenue, Box 0131, San Francisco, CA 94143, USA.
Joan F. Hilton, Department of Epidemiology and Biostatistics, University of California, San Francisco, 550 16th Street, San Francisco, CA 94158, USA.
Lisa Shieh, Department of Medicine, Stanford University, 300 Pasteur Drive, Stanford, CA 94305, USA.
Jason Satterfield, Department of Medicine, University of California, San Francisco, USA, 1701 Divisadero Street, Suite 500, San Francisco, CA 94115 (415) 353-2104.
References
- 1.Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312(13):1295–1296. doi: 10.1001/jama.2014.10186. [DOI] [PubMed] [Google Scholar]
- 2.Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015 Jul; doi: 10.1016/j.pec.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1982. 1999;49(5):651–661. doi: 10.1016/s0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 5.Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. What is a medical decision? A taxonomy based on physician statements in hospital encounters: a qualitative study. BMJ Open. 2016;6(2):e010098. doi: 10.1136/bmjopen-2015-010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler FJ, Levin CA, Sepucha KR. Informing and involving patients to improve the quality of medical decisions. Health Aff Proj Hope. 2011;30(4):699–706. doi: 10.1377/hlthaff.2011.0003. [DOI] [PubMed] [Google Scholar]
- 7.Weiner SJ, Kelly B, Ashley N, et al. Content coding for contextualization of care: evaluating physician performance at patient-centered decision making. Med Decis Mak Int J Soc Med Decis Mak. 2014;34(1):97–106. doi: 10.1177/0272989X13493146. [DOI] [PubMed] [Google Scholar]
- 8.Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573–579. doi: 10.7326/0003-4819-158-8-201304160-00001. [DOI] [PubMed] [Google Scholar]
- 9.Matthias MS, Salyers MP, Frankel RM. Re-thinking shared decision-making: context matters. Patient Educ Couns. 2013;91(2):176–179. doi: 10.1016/j.pec.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Clayman ML, Bylund CL, Chewning B, Makoul G. The Impact of Patient Participation in Health Decisions Within Medical Encounters: A Systematic Review. Med Decis Mak. 2016;36(4):427–452. doi: 10.1177/0272989X15613530. [DOI] [PubMed] [Google Scholar]
- 11.Shay LA, Lafata JE. Understanding patient perceptions of shared decision making. Patient Educ Couns. 2014;96(3):295–301. doi: 10.1016/j.pec.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9–18. doi: 10.1016/j.pec.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709–e718. doi: 10.3399/bjgp14X682297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joosten EaG, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CPF, de Jong CaJ. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219–226. doi: 10.1159/000126073. [DOI] [PubMed] [Google Scholar]
- 15.Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 16.Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care J Int Soc Qual Health Care ISQua. 2011;23(3):269–277. doi: 10.1093/intqhc/mzr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammed K, Nolan MB, Rajjo T, et al. Creating a Patient-Centered Health Care Delivery System: A Systematic Review of Health Care Quality From the Patient Perspective. Am J Med Qual Off J Am Coll Med Qual. 2014 Jul; doi: 10.1177/1062860614545124. [DOI] [PubMed] [Google Scholar]
- 18.Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548–555. doi: 10.1136/bmjqs-2012-001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526–535. doi: 10.1016/j.pec.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Frosch DL, May SG, Rendle KAS, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled “difficult” among key obstacles to shared decision making. Health Aff Proj Hope. 2012;31(5):1030–1038. doi: 10.1377/hlthaff.2011.0576. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SK, Bautista CA, Hong SY, Weissfeld L, White DB. An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care units. Am J Respir Crit Care Med. 2011;183(7):915–921. doi: 10.1164/rccm.201008-1214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240–246. doi: 10.1016/j.pec.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 23.Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275–280. doi: 10.1177/0272989X03256006. [DOI] [PubMed] [Google Scholar]
- 24.Légaré F, Bekker H, Desroches S, et al. How can continuing professional development better promote shared decision-making? Perspectives from an international collaboration. Implement Sci IS. 2011;6:68. doi: 10.1186/1748-5908-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301–312. doi: 10.1016/j.pec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Moumjid N, Gafni A, Brémond A, Carrère M-O. Shared decision making in the medical encounter: are we all talking about the same thing? Med Decis Mak Int J Soc Med Decis Mak. 2007;27(5):539–546. doi: 10.1177/0272989X07306779. [DOI] [PubMed] [Google Scholar]
- 27.Hallström I, Elander G. Decision-making during hospitalization: parents’ and children’s involvement. J Clin Nurs. 2004;13(3):367–375. doi: 10.1046/j.1365-2702.2003.00877.x. [DOI] [PubMed] [Google Scholar]
- 28.Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. Temporal characteristics of decisions in hospital encounters: a threshold for shared decision making? A qualitative study. Patient Educ Couns. 2014;97(2):216–222. doi: 10.1016/j.pec.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Baumeister RF, Leary MR. Writing narrative literature reviews. Rev Gen Psychol. 1997;1(3):311. [Google Scholar]
- 30.Moody DL. Theoretical and practical issues in evaluating the quality of conceptual models: current state and future directions. Data Knowl Eng. 2005;55(3):243–276. doi: 10.1016/j.datak.2004.12.005. [DOI] [Google Scholar]
- 31.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351–377. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- 32.Basics of Qualitative Research. SAGE Publications Inc; [Accessed September 13, 2016]. https://us.sagepub.com/en-us/nam/basics-of-qualitative-research/book235578. [Google Scholar]
- 33.Halley MC, Rendle KA, Frosch DL. A conceptual model of the multiple stages of communication necessary to support patient-centered care. J Comp Eff Res. 2013;2(4):421–433. doi: 10.2217/cer.13.46. [DOI] [PubMed] [Google Scholar]
- 34.Torke AM, Petronio S, Sachs GA, Helft PR, Purnell C. A conceptual model of the role of communication in surrogate decision making for hospitalized adults. Patient Educ Couns. 2012;87(1):54–61. doi: 10.1016/j.pec.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falzer PR, Garman MD. A conditional model of evidence-based decision making: Model of evidence-based decision making. J Eval Clin Pract. 2009;15(6):1142–1151. doi: 10.1111/j.1365-2753.2009.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holzmueller CG, Wu AW, Pronovost PJ. A framework for encouraging patient engagement in medical decision making. J Patient Saf. 2012;8(4):161–164. doi: 10.1097/PTS.0b013e318267c56e. [DOI] [PubMed] [Google Scholar]
- 37.Elwyn G, Lloyd A, May C, et al. Collaborative deliberation: a model for patient care. Patient Educ Couns. 2014;97(2):158–164. doi: 10.1016/j.pec.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Ruland CM, Bakken S. Developing, implementing, and evaluating decision support systems for shared decision making in patient care: a conceptual model and case illustration. J Biomed Inform. 2002;35(5–6):313–321. doi: 10.1016/S1532-0464(03)00037-6. [DOI] [PubMed] [Google Scholar]
- 39.Shepperd S, Charnock D, Gann B. Helping patients access high quality health information. Br Med J. 1999;319(7212):764. doi: 10.1136/bmj.319.7212.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Légaré F, Stacey D, Pouliot S, et al. Interprofessionalism and shared decision-making in primary care: a stepwise approach towards a new model. J Interprof Care. 2011;25(1):18–25. doi: 10.3109/13561820.2010.490502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coutu M-F, Légaré F, Durand M-J, et al. Operationalizing a Shared Decision Making Model for Work Rehabilitation Programs: A Consensus Process. J Occup Rehabil. 2015;25(1):141–152. doi: 10.1007/s10926-014-9532-7. [DOI] [PubMed] [Google Scholar]
- 42.Hölzel LP, Kriston L, Härter M. Patient preference for involvement, experienced involvement, decisional conflict, and satisfaction with physician: a structural equation model test. BMC Health Serv Res. 2013;13(1):1. doi: 10.1186/1472-6963-13-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curtis JR, White DB. PRactical guidance for evidence-based icu family conferences. Chest. 2008;134(4):835–843. doi: 10.1378/chest.08-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks, Silverman L, Wallen G. Shared Decision Making: A Fundamental Tenet in a Conceptual Framework of Integrative Healthcare Delivery. Integr Med Insights. 2013 Sep;:29. doi: 10.4137/IMI.S12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Müller-Engelmann M, Donner-Banzhoff N, Keller H, et al. When decisions should be shared: a study of social norms in medical decision making using a factorial survey approach. Med Decis Mak Int J Soc Med Decis Mak. 2013;33(1):37–47. doi: 10.1177/0272989X12458159. [DOI] [PubMed] [Google Scholar]
- 46.Mccaffery KJ, Shepherd HL, Trevena L, et al. Shared decision-making in Australia. Z Für Ärztl Fortbild Qual. 2007;101(4):205–211. doi: 10.1016/j.zgesun.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 47.Rubin MA. The Collaborative Autonomy Model of Medical Decision-Making. Neurocrit Care. 2014;20(2):311–318. doi: 10.1007/s12028-013-9922-2. [DOI] [PubMed] [Google Scholar]
- 48.McCullough LB. The professional medical ethics model of decision making under conditions of clinical uncertainty. Med Care Res Rev. 2012 doi: 10.1177/1077558712461952. doi:1077558712461952. [DOI] [PubMed] [Google Scholar]
- 49.Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. Milbank Q. 2009;87(2):368–390. doi: 10.1111/j.1468-0009.2009.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore JE, Titler MG, Kane Low L, Dalton VK, Sampselle CM. Transforming Patient-Centered Care: Development of the Evidence Informed Decision Making through Engagement Model. Womens Health Issues. 2015;25(3):276–282. doi: 10.1016/j.whi.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med 1982. 1997 Mar;44(5):681–92. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 52.Stacey D, Légaré F, Pouliot S, Kryworuchko J, Dunn S. Shared decision making models to inform an interprofessional perspective on decision making: a theory analysis. Patient Educ Couns. 2010;80(2):164–172. doi: 10.1016/j.pec.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Epstein RM, Gramling RE. What is shared in shared decision making? Complex decisions when the evidence is unclear. Med Care Res Rev MCRR. 2013;70(1 Suppl):94S–112S. doi: 10.1177/1077558712459216. [DOI] [PubMed] [Google Scholar]
- 54.Kon AA. The shared decision-making continuum. JAMA. 2010;304(8):903–904. doi: 10.1001/jama.2010.1208. [DOI] [PubMed] [Google Scholar]
- 55.Rapley T. Distributed decision making: the anatomy of decisions-in-action. Sociol Health Illn. 2008;30(3):429–444. doi: 10.1111/j.1467-9566.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- 56.Braddock CH, Fihn SD, Levinson W, Jonsen AR, Pearlman RA. How doctors and patients discuss routine clinical decisions. Informed decision making in the outpatient setting. J Gen Intern Med. 1997;12(6):339–345. doi: 10.1046/j.1525-1497.1997.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braddock CH, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. JAMA. 1999;282(24):2313–2320. doi: 10.1001/jama.282.24.2313. [DOI] [PubMed] [Google Scholar]
- 58.Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups. Soc Sci Med 1982. 2009;69(12):1805–1812. doi: 10.1016/j.socscimed.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 59.Towle A, Godolphin W, Grams G, Lamarre A. Putting informed and shared decision making into practice. Health Expect Int J Public Particip Health Care Health Policy. 2006;9(4):321–332. doi: 10.1111/j.1369-7625.2006.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Légaré F, Stacey D, Gagnon S, et al. Validating a conceptual model for an interprofessional approach to shared decision making: a mixed methods study. J Eval Clin Pract. 2011;17(4):554–564. doi: 10.1111/j.1365-2753.2010.01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]