Abstract
The application of a cross-linker to demineralized dentin is reportedly effective at extending the durability of dentin bonds. Yet, results for the fatigue crack growth resistance have been limited to only a three-step etch-and-rinse adhesive.
Objective
To compare the effect of a cross- linker pretreatment on the durability of resin-dentin bonds prepared with a two- vs three-step adhesive system, in terms of the fatigue crack growth resistance.
Methods
Bonded interface Compact Tension (CT) specimens were prepared using commercial two- and three-step etch-and-rinse adhesives and compatible hybrid resin-composite. For the treated groups, adhesive bonding was preceded by a 1 min application of an experimental carbodiimide (EDC) conditioner to the acid-etched dentin. The control groups received no such treatment. The fatigue crack growth resistance was examined after storage in phosphate-buffered artificial saliva 37° C for 0, 3 and 6 months.
Results
There was no significant difference in the immediate fatigue crack growth resistance of the control or EDC-treated groups at 0 months for either adhesive system. After 3 and 6 months of storage, the EDC-treated groups exhibited significantly greater (p≤0.05) fatigue crack growth resistance than the controls. Although the EDC treatment was equally effective in deterring degradation for both adhesives, bonds prepared with the three-step system exhibited the lowest resistance to fatigue crack growth overall.
Significance
An EDC treatment applied during dentin bonding could help maintain the durability of bonds prepared with two or three-step adhesive bonding systems.
Keywords: collagen, cross-linker, dentin bonding agents, durability, EDC, endogenous proteinases, fatigue crack growth, fracture
INTRODUCTION
Over the past few decades the quality of adhesive bonds achieved to dentin and enamel have increased substantially. Although most commercial products are capable of achieving acceptable immediate bond strength to the tooth, adhesive bonds to dentin undergo a gradual reduction in durability over time [1–5]. The solution to this problem has been elusive as both the degree of degradation and the contributing mechanisms are not consistent for all adhesive systems [6–9]. Clinically relevant approaches for increasing the durability of adhesive bonds to the tooth are essential to the success of adhesive dentistry.
One of the primary forms of degradation to resin-dentin adhesive bonds is the exposure and activation of endogenous dentin proteases [10,11]. Contemporary bonding procedures involving either etch-and-rinse or self-etch adhesives cause activation of matrix-metalloproteinases (MMP)s and cysteine cathepsins [e.g. 11–14]. These are host-derived proteolytic enzymes that are bound to the dentin collagen matrix. When uncovered by etching, the MMPs slowly solubilize the collagen fibrils [10] and remain active even after resin-infiltration. This process triggers the gradual destruction of poorly infiltrated fibrils within the hybrid layers [15–17]. Resin tags that are not well anchored to collagen fibrils and/or poor collagen integrity are critical weak links of the bonded interface [18]. The degradation of collagen matrices resulting from endogenous protease activity can cause a decrease in bond strength over time and a reduction in the durability of resin-dentin bonds.
One promising approach to extending the durability of resin-dentin bonds is to treat acid-etched dentin with cross-linking agents that are applied just prior to the adhesive. Various cross-linking agents have been explored for this purpose such as glutaraldehyde, genipin, proanthrocyanidin, and carbodiimide [19–21]. Cross-linking of the fibrils serves to inactivate the catalytic site of proteases [22], which reduces the susceptibility of the collagen fibrils to enzymatic degradation by collagenases.
Carbodiimide (hereafter referred to as EDC) has recently been identified as one of the most promising cross-linkers for stabilizing dentin bonds. In comparison to other products it has the advantage of very low cytotoxicity, and an ability to preserve dentin bond strength within clinically acceptable treatment times [23–26]. The application of 0.5 M EDC for 1 min during dentin bonding reportedly reduced the degradation in fatigue crack growth resistance of bonds prepared with a three-step resin adhesive bonding system [27]. While promising, two-step adhesive systems generally exhibit lower durability and resistance to degradation with aging [15]. Indeed, EDC was found less effective in maintaining the microtensile strength of dentin bonds prepared with a two-step adhesive system when compared to results obtained with a comparable three-step system [26].
A comparison of the effectiveness of EDC treatment in preserving the fatigue crack growth resistance of dentin bonds prepared using two- and three-step adhesive systems has not been reported. Therefore, the primary objective of this investigation was to compare the effectiveness of an EDC treatment (consisting of 0.5 M and 1 min exposure) in maintaining the fatigue crack growth resistance of adhesive bonds to dentin prepared with a two-step vs. three-step adhesive resin after aging. The null hypotheses to be tested were that (1) there is no difference in the fatigue crack growth behavior of dentin bonds prepared with two- vs. three-step adhesive resins, and (2) there is no difference in the effectiveness of the EDC treatment on the fatigue crack growth resistance for the two resin systems up to 6 months of storage.
MATERIALS AND METHODS
The fatigue crack growth resistance of resin-dentin bonds was evaluated using specimens that were prepared from sections of mid-coronal dentin. Longitudinal sections were obtained from caries-free human third molars, which were acquired with informed signed consent from participating clinics in Maryland with record of age (18≤age≤30 yrs) according to an approved protocol (#Y04DA23151). Each tooth was sectioned using a slicer/grinder (Chevalier Smart-H818II, Chevalier Machinery, Santa Fe Springs, CA, USA) with diamond abrasive slicing wheels (#320 mesh abrasives) and copious water coolant. Two mm thick sections were obtained from the mid coronal region as shown in Figure 1a. The remaining materials used in the development of the specimens included a three-step etch-and-rinse adhesive (Scotchbond Multipurpose, SBMP, 3M ESPE) and a two-step etch-and-rinse adhesive (Sinlge Bond, SB, 3M ESPE), as well as a compatible resin composite (Z100, 3M ESPE).
Figure 1.
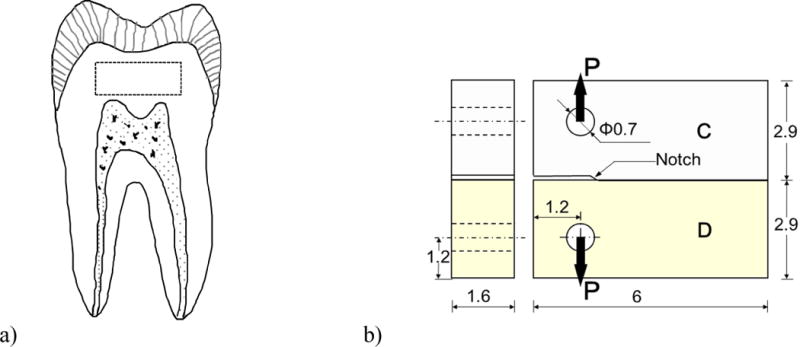
Schematic diagrams describing a) the location and orientation of coronal dentin sections obtained for the bonded interface specimens (outlined by the dashed line), and b) the inset Compact Tension (CT) specimen geometry for characterizing the fatigue crack growth resistance. All dimensions are listed in millimeters. The diagram highlights the dentin (D) and resin composite (C), and orientation of the opening mode load (P). Adhesive bonding to the dentin sections was always performed on the edge facing the dentin enamel junction using either SB or SBMP adhesive. The notch was created by insertion of a Mylar strip.
Bonded interface Compact Tension (CT) specimens were prepared from the sections of dentin using a special molding technique described previously [18, 28]. As noted in Figure 1b, the dentin sections represented half of the completed CT specimen geometry. The outer dentin (edge oriented farthest from the pulp) was etched for 15 sec with SB 37% phosphoric acid etchant and rinsed with water for 15 sec. For SBMP, the primer and adhesive were applied to the etched surface according to the manufacturer’s recommendations. Similarly, for the SB specimens the resin adhesive was applied and light-cured according to the manufacturer’s instructions. Thereafter, the prepared sections were placed in a specially designed mold for incremental application of the resin composite. A thin Mylar sheet was placed at one end of the interface to introduce a molded notch as shown in Figure 1b. The composite was cured on both sides for 40 sec using a quartz–tungsten–halogen light-curing unit (Demetron VCL 401, Demetron, CA, USA) with output intensity of 600 mW/cm^2 and with tip diameter wider than 10 mm. Following this procedure the specimens were placed within a phosphate-buffered artificial saliva at 37°C until further evaluation. The solution contained 0.2% sodium azide to prevent microbial growth.
The durability of the bonded interfaces was evaluated in two conditions, namely with an experimental treatment formulated to inactivate endogenous dentin proteases, and without such treatment (control). For the treated specimens, application of the adhesive was preceded by conditioning the demineralized collagen using an experimental aqeous solution of 0.5 M ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) for 60 sec. The specimens were then rinsed with water (15 sec) and then lightly blotted. The remainder of the specimen preparation process was identical to that for those specimens without EDC.
The bonded sections were released from the mold and lightly sanded on both sides. Two holes were introduced using a miniature milling machine to facilitate the opening mode loading as evident in Figure 1b. Detailed procedures used for specimen preparation have been presented previously [28].
For each resin-adhesive system, a total of 36 specimens were prepared including an equal number of cross-linked specimens (treated with EDC) and non-cross-linked controls. The fatigue crack growth resistance of the specimens was evaluated after a storage period of 0, 3 or 6 months with six specimens in each cell (treatment and time). Those specimens evaluated at 0 months represent the “immediate” fatigue crack growth resistance (i.e. without storage) and were tested after a period of at least 48 hours in phosphate-buffered saline from the date of preparation.
The CT specimens were subjected to cyclic Mode I loading [29] using a universal testing system (EnduraTEC Model ELF 3200, Minnetonka, MN, USA) with load capacity and sensitivity of 225 N and ±0.01 N, respectively. All experiments were performed within a bath of Hanks Balanced Salt Solution (HBSS) at room temperature (pH 7.4). The loading was applied under load control actuation, a frequency of 5 Hz and stress ratio (R) of 0.1. Crack length measurements were acquired using an imaging system that consisted of a microscope (Optem zoom 70xl 391940, QIOPTIQ, Luxembourg) and CCD camera. Sequential measurements of the crack length were used to estimate the incremental crack extension (Δa) as a function of the number of loading cycles in the increment (ΔN). The increment of cyclic loading ranged from 5 ≤ΔN≤30k cycles and the length of crack extension generally ranged from 0.02≤Δa≤0.15mm. Cyclic loading was continued until the specimen underwent complete fracture. These procedures have been used previously for evaluating the resin-dentin bonded interface [18, 27] and the fatigue crack growth resistance of dentin [29, 30].
The fatigue crack growth experiments performed with each specimen provided measurements of crack length and corresponding number of loading cycles. The data was used to quantify the incremental crack growth rate (Δa/ΔN) and the corresponding stress intensity range (ΔK) over the range of cyclic crack extension. The stress intensity range for the inset CT specimens was estimated as a function of crack length according to
| (1) |
where ΔP is the opening load range (Pmax – Pmin), B is the thickness of the specimen, W is the distance between the center of the loading points and free boundary in front of the crack, and α = a/W is the ratio of the average crack length to the in-plane specimen width (Fig. 1b). For each specimen, the incremental crack growth rate was then plotted in terms of the stress intensity range to further evaluate the fatigue crack response.
According to the distribution of data, the threshold stress intensity range (ΔKth) was estimated at the onset of cyclic crack growth, and represents the minimum stress intensity necessary for cyclic crack extension. The steady-state portion of the fatigue crack growth responses was also identified and modeled using the Paris Law [31] according to
| (2) |
where C and m are the fatigue crack growth coefficient and exponent, respectively. The average values of these two parameters were determined for each group of specimens. Significant differences in the fatigue crack growth parameters resulting from EDC treatment and storage time were identified using a two-way analysis of variance (ANOVA) with Tukey’s HSD; significance was identified by p≤0.05. The fatigue crack growth distributions were compared to establish significant differences between the groups using the Wilcoxon Rank Sum test with the critical value (alpha) set at 0.05.
Selected fractured specimens were evaluated using scanning electron microscopy (SEM) in secondary electron imaging mode with a commercial instrument (FEI Model Nova NanoSEM 450; Hillsboro, OR, USA). Prior to the SEM analysis, the specimens were dehydrated in an ascending ethanol series (70–100%), fixed in hexamethyldisilazane, and then sputtered with gold/palladium to enhance the conductivity of the dentin and resin adhesive. The fracture surfaces were inspected to identify any potential mechanisms of degradation evident or other factors contributing to the fatigue crack growth behavior.
RESULTS
Fatigue crack growth responses for the interface specimens prepared with EDC treatment are shown in Figure 2. Specifically, Figure 2a shows the results for a representative specimen prepared with SB adhesive, and evaluated without storage in artificial saliva (i.e. at 0 months). This cyclic crack growth history identifies the threshold stress intensity range (ΔKth) defined at the onset of incremental crack growth, as well as the Paris Law exponent, which quantitates the slope of the steady-state response. The fatigue crack growth responses for all of the specimens evaluated without storage, including the control group, are shown in Figure 2b. Similarly, results for all of the specimens prepared with EDC and SBMP adhesive and evaluated at 0 months are shown in Figure 2c. According to the Wilcoxon Rank Sum test, there was no significant difference between the control and EDC-treated specimens prepared with SB (Z = 2.1622; p=0.098) or SBMP (Z = −0.127; p=0.899) adhesive.
Figure 2.
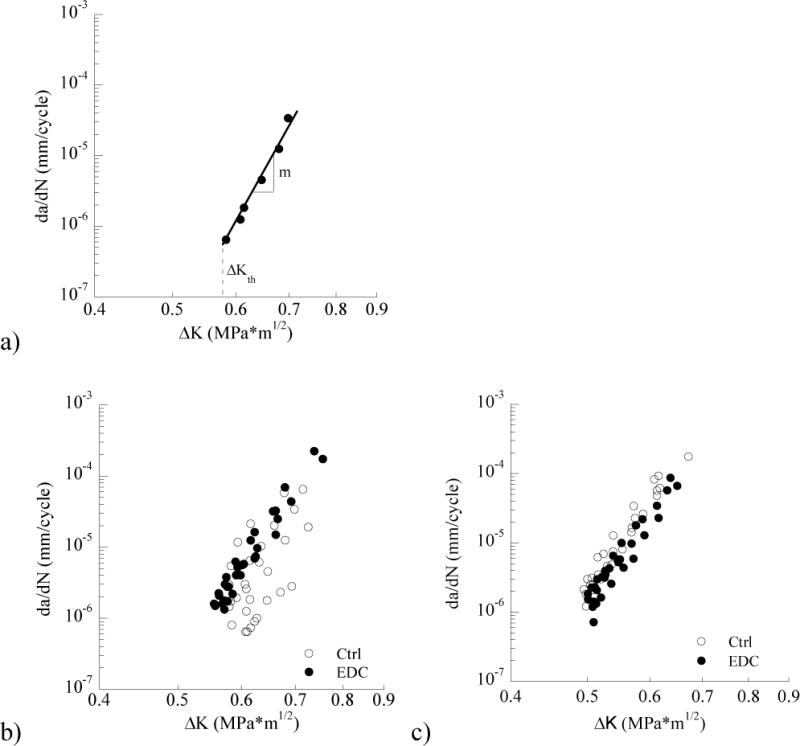
Fatigue crack growth responses for specimens that were evaluated without long term storage (i.e. at 0 months). a) a representative response for an EDC treated specimen prepared with SB adhesive. Highlighted in this figure are the stress intensity threshold (ΔKth) and the Paris Law exponent (m). The Paris Law coefficient (C, not shown) is the y-intercept of the steady-state response curve (i.e. at ΔK = 1 MPa•m0.5). b) cumulative responses for the control and EDC specimens prepared with SB adhesive and evaluated at 0 months. c) cumulative responses for the control and EDC specimens prepared with SBMP adhesive and evaluated at 0 months.
The average values of the fatigue crack growth parameters for the SB and SBMP specimens, including the ΔKth and the Paris Law parameters (m, C), are listed in Tables 1 and 2, respectively. Note that only the mean is presented for the crack growth coefficient (C) due to the comparatively large variation in this parameter. There was no significant difference in the average ΔKth between the control and EDC-treated groups for either adhesive system when evaluated immediately after bonding. Similarly, there was no significant difference (p>0.05) in the Paris Law parameters (m, C) between the control and treated groups for the two different resin adhesives prior to storage.
Table 1.
The average values for the fatigue crack growth parameters including the threshold stress intensity range (ΔKth) and Paris Law constants (m, C)). Results are presented for the specimens receiving EDC cross-linking treatment during bonding (EDC) and those that did not (Control) at the three periods of storage.
| SB adhesive system | 0 months | 3 months | 6 months | |||
|---|---|---|---|---|---|---|
| Property | Control | EDC | Control | EDC | Control | EDC |
| ΔKth [MPa•m05] | a0.58±0.05 | a0.54±0.1 | b0.51±0.02 | a0.53±0.02 | b0.51±0.02 | a0.54±0.02 |
| M | a17.5±1.1 | a17.0±1.3 | b17.4±2.6 | a16.7±2.0 | b19.5±2.5 | a17.2±1.5 |
| C [(mm/cycle)(MPa•m05) −m] | a2.2 × 10−2 | a4.2 × 10−2 | b5.3 × 10−2 | a7.1 × 10−2 | b123 × 10−2 | a7.6 × 10−2 |
| SBMP adhesive system | 0 months | 3 months | 6 months | |||
|---|---|---|---|---|---|---|
| Property | Control | EDC | Control | EDC | Control | EDC |
| ΔKth [MPa•m05] | a0.50±0.02 | a0.51±0.02 | a0.47±0.03 | a0.48±0.03 | a0.45±0.02 | a0.49±0.03 |
| m | a16.0±0.6 | a16.8±0.2 | a17.4±0.5 | a15.8±1.3 | b21.5±2.8 | a14.1±3.2 |
| C [(mm/cycle)(MPa•m05) −m] | a15.4 × 10−2 | a6.9 × 10−2 | a68.3 × 10−2 | a5.9×10−2 | b1410 × 10−2 | a6.2 × 10−2 |
The fatigue crack growth responses for the control specimens (prepared without EDC treatment) after storage in artificial saliva are shown in Figure 3. The responses for the specimens prepared with SB and SBMP adhesive are shown in Figures 3a and 3b, respectively. For both adhesive resins, there is a shift in the fatigue crack growth data to the left with increasing storage time that signifies a decrease in the resistance to cyclic crack growth. According to the Wilcoxon Rank Sum test, the specimens prepared with SB adhesive exhibited significantly lower resistance to fatigue crack growth after 3 months (Z = 2.506; p=0.0122) and to a larger extent after 6 months (Z = 3.178; p=0.0015). Similarly, the specimens prepared with SBMP exhibited significantly lower resistance to fatigue crack growth after 3 months (Z = 3.242; p=0.0012) and after 6 months (Z = 5.192; p=0.000). The average fatigue crack growth parameters for these SB and SBMP specimens are listed in Tables 1 and 2, respectively.
Figure 3.
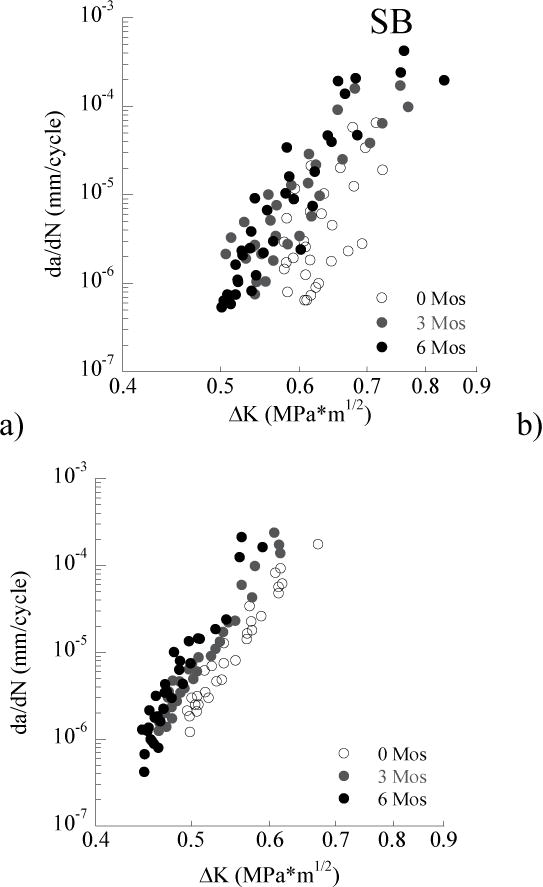
Cumulative fatigue crack growth responses for the control and treated groups prepared with the SB adhesive after (a) 3 months and (b) 6 months of aging.
A comparison of the responses for the specimens treated with EDC as a function of storage time is shown in Figure 4. Specifically, results for the SB and SBMP adhesives are shown in Figures 4a and 4b, respectively. There was no significant reduction in the fatigue crack growth resistance of the SB specimens, after 6 months storage with respect to immediately after bonding (Z = 1.7385; p=0.0821). Similarly, there was no significant change in the fatigue crack growth resistance of the SBMP specimens with respect to those samples evaluated without storage (Z = 1.4445; p=0.1486).
Figure 4.
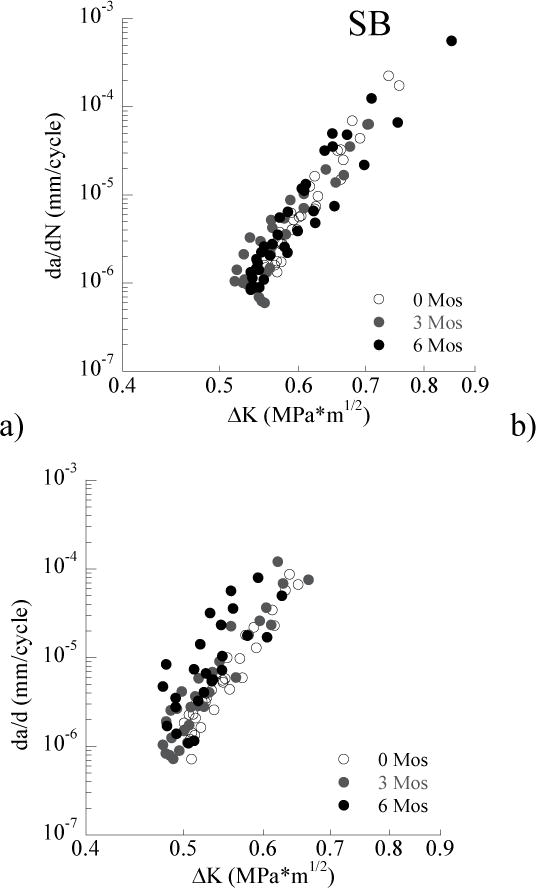
A comparison of the fatigue crack growth resistance and changes with aging for the control specimens (without EDC) for both adhesive systems. a) SB, b) SBMP
Results for the fatigue crack growth parameters obtained from experiments performed on the individual specimens from each group (involving treatment and storage) were pooled to estimate the averages. These values were utilized to model the mean cyclic crack growth rates after storage for 6 months (Figure 5). As evident from these distributions, the specimens prepared with SBMP exhibited the lowest resistance to fatigue crack growth in both the control and EDC treated conditions. Both systems underwent a clear reduction in the average rate of cyclic crack extension with EDC treatment. However, the EDC treatment appeared to introduce a larger degree of improvement to the fatigue crack growth resistance of the SB specimens.
Figure 5.
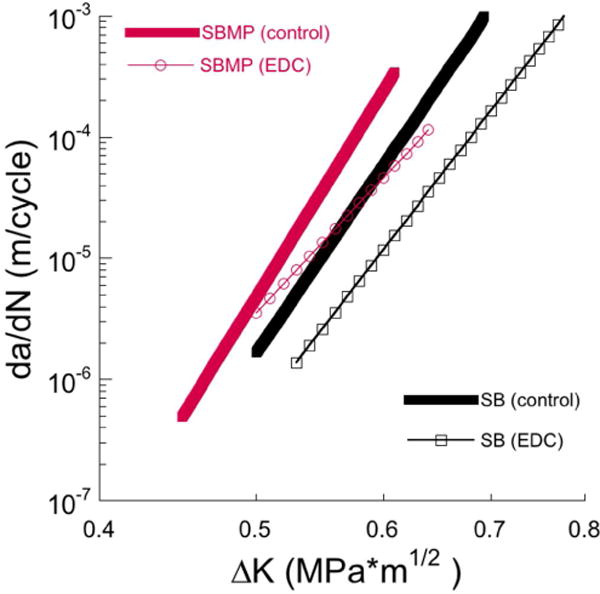
A comparison of the degradation in the fatigue crack growth resistance with aging for the specimens treated with EDC for both adhesive systems. a) SB, b) SBMP
Micrographs of the fracture surfaces from representative samples are shown in Figure 6. The views in this figure show the dentin side of the fracture surface after failure of the interface and separation of the resin composite. In general, the fracture surfaces largely consisted of adhesive failure involving the resin-adhesive or hybrid layer; fatigue crack growth did not occur within the resin composite in any of the samples evaluated. Rather, cyclic crack extension occurred primarily within or at the bottom of the hybrid layer, where the resin tags penetrate into the underlying mineralized dentin. Micrographs obtained from the fracture surfaces of control and EDC treated SB specimens evaluated at 0 months are shown in Figures 6a and 6b, respectively. The features evident in these two micrographs appear very consistent, despite the application of EDC to the sample in Figure 6b. There is a small degree of debonding between some of the tags and surrounding intertubular dentin, which is equally evident in the control and EDC-treated specimens.
Figure 6.
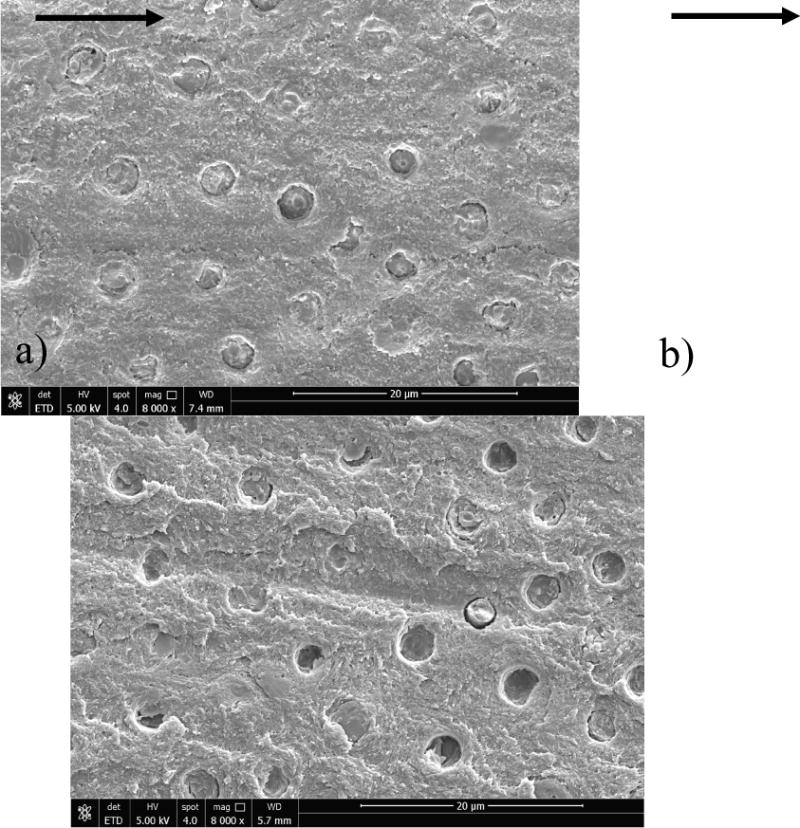
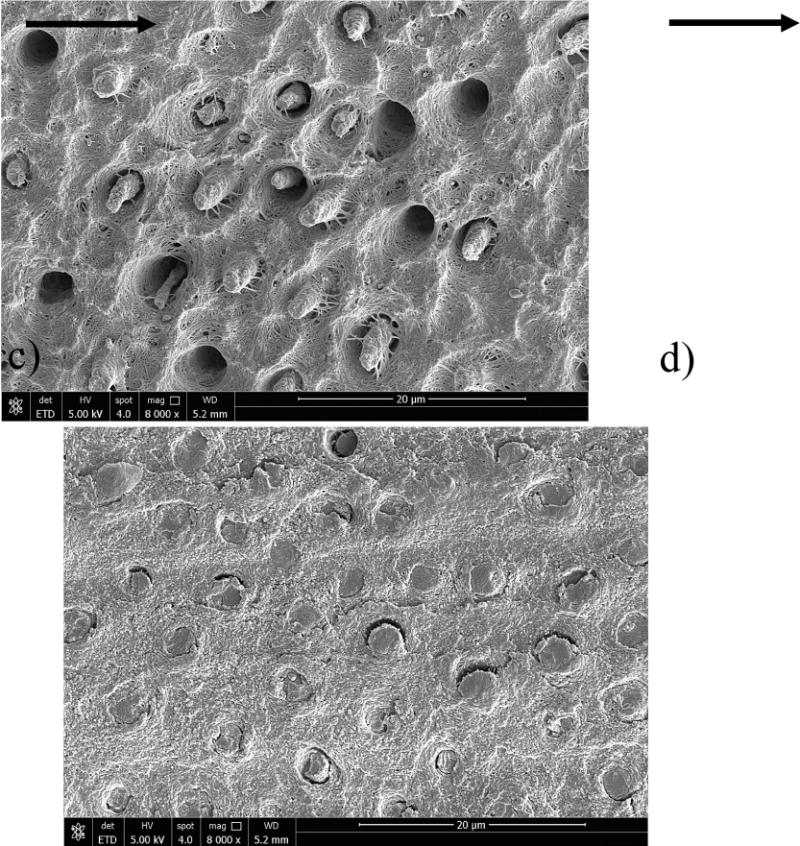
Micrographs obtained from SEM analysis of the fracture surfaces resulting from fatigue crack growth in the SB specimens. Fracture surfaces from representative specimens evaluated after 0 months storage are shown for a) SB control and b) SBw/EDC. Similarly, the fracture surfaces from representative specimens evaluated after 6 months are shown for c) SB control and d) SB w/EDC. The orientation of fatigue crack growth in all of the samples was from left to right. The overlying hybrid layer had a thickness equivalent to about two tubule diameters. Note the exposed collagen fibrils and evidence of resin table pullout in (c).
Micrographs from the fracture surfaces of representative control and EDC-treated SB specimens evaluated after 6 months of storage are shown in Figure 6c and 6d, respectively. For the control specimen in Figure 6c, there is evidence of resin tag pullout from the tubule lumen, as well as fractured tags protruding above the remaining surface. The most marked feature of this surface is the abundance of exposed collagen fibrils that extend from the intertubular regions to the remnant tags. Contrary to the fracture surfaces of the tags in the other micrographs, those in Figure 6c appear to be degraded and have undergone a reduction in diameter. The surfaces of specimens in the EDC groups evaluated after 3 (not shown) and 6 months of storage (Fig. 6d) appeared very similar to those at 0 months. There was no evidence of degradation or indications of changes in the mechanisms of fatigue crack growth. The fracture surface morphology for the specimens prepared with SBMP adhesive (not shown) were very consistent with the characteristics of the SB specimens and have been presented elsewhere [27].
Figure 7 presents additional high magnification views of the fracture surfaces resulting from fatigue crack growth in the control specimens that were evaluated after 6 months storage. In particular, the fracture surface of the SB specimen presented in Figure 6c is shown at greater magnification in Figure 7a. This micrograph provides details the interface between the resin tags and interpenetrating collagen fibrils. A similar view for a control specimen prepared with SBMP adhesive is shown in Figure 7b. For both resin systems there are sparse collagen fibrils extending across the interface of the intertubular matrix to the penetrating tags. This suggests that all other collagen fibrils that anchored the resin tags had dissolved. Some of the residual fibrils appear degraded as evident from the smaller diameter (white arrows). There are also fractured fibrils at the boundary of the tags, and most evident in the SBMP sample of (Fig. 7b).
Figure 7.
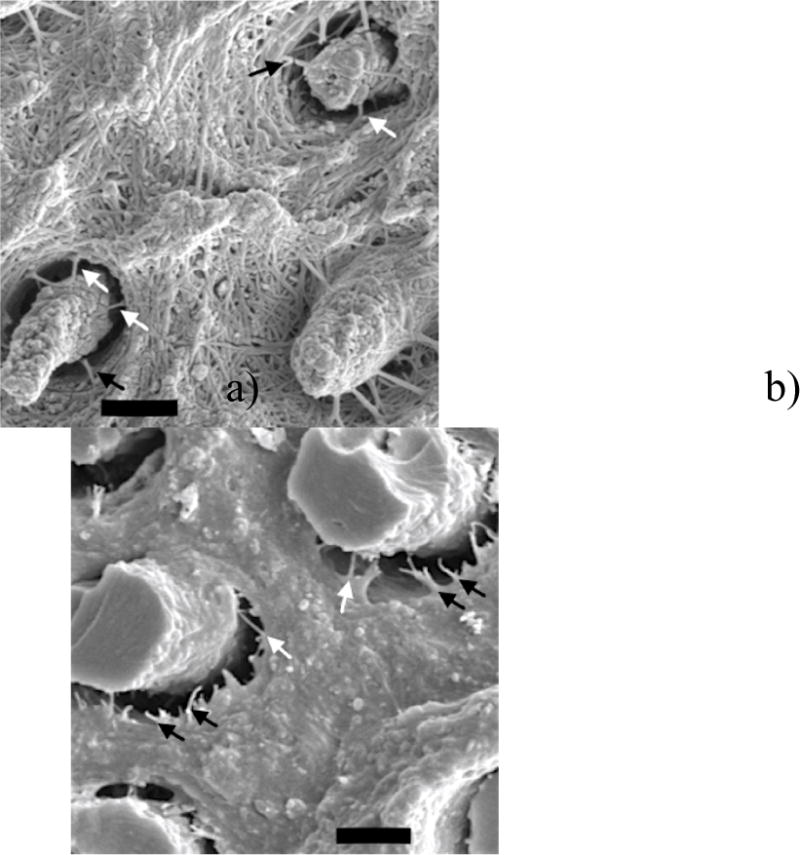
High magnification SEM micrographs of the fracture surfaces resulting from fatigue crack growth in a) control SB and b) control SBMP after 6 months of aging. Scale bar represents 2.5 μm. Arrows highlight fractured collagen fibrils (black) and degraded fibrils (white).
DISCUSSION
When the fatigue crack growth resistance of the bonded interfaces was evaluated without aging, there was no difference between the control and EDC treated conditions for either resin system (Figure 2). Therefore, the cross-linking treatment did not degrade the resistance to the initiation and growth of cracks under cyclic loading. Bonds prepared with the two-step system resin (Figure 2a) possessed significantly greater resistance to fatigue crack growth overall. That statement is true for both the control (Z=5.163, p=0.000) and EDC treated specimens (Z=4.783, p=0.000). Therefore, the first null hypothesis must be rejected.
The initiation of cyclic crack extension was quantified in terms of the stress intensity threshold (ΔKth) and the steady-state growth rate in terms of the Paris Law parameters (m and C). There are three aspects of degradation that can be identified in the bonded interface durability with storage time using those parameters. The reduction in ΔKth with storage for the control signifies a decrease in resistance to the initiation of cyclic crack growth from flaws along the bonded interface. This reduction occurred within only 6 months of aging and is expected to continue with additional time. Furthermore, there was an increase in m and C with storage time, which denote an increase in sensitivity to the presence of cracks and an increase in the incremental crack growth rate, respectively. The growth rate increased from 1 to 8X after only 6 months in the interfaces prepared without EDC (Figure 5). Hence, the EDC treatment is expected to promote a substantial increase in the life of the restoration, regardless of the adhesive.
Two-step adhesive systems are considered less durable in comparison to three-step systems, and more susceptible to enzymatic degradation [15]. Yet, the interfaces prepared with the three-step system exhibited inferior fatigue crack growth resistance. These results conflict with those for the microtensile strength of dentin bonds after aging [25] where the crosslinking treatment (0.3 M EDC for 1 min) was less effective in resisting degradation of the two-step system. There is additional evidence from previous in vitro [9] and in vivo [2] studies suggesting that three-step adhesives exhibit superior durability as well, which has been attributed to their greater hydrophobicity [32] and curing ability [15,33]. Evidently the mechanisms contributing to the strength of dentin bonds are not equivalent to those that resist fatigue crack growth.
After aging, the EDC treated specimens required larger cyclic stress intensity to propagate fatigue cracks along the bonded interfaces. Using a value of 0.6 MPa•m0.5 to represent the driving force contributing to crack growth in restored teeth [34], the EDC treatment reduced the rate of cyclic extension in the bonded interfaces by at least 5X. That would support rejection of the 2nd null hypothesis. However, there was no reduction in the fatigue crack growth resistance of the EDC-treated resin-dentin bonds for either adhesive system after the 6-month storage period (Figure 4). Therefore, the second null hypothesis must be accepted.
Characteristics of the fracture surfaces from the SB and SBMP samples that were not subjected to aging appeared very consistent. Detailed views of the SBMP samples were presented previously [27] and are very consistent with the SB results. Failure of the resin tags occurred consistently at the primary fracture plane without evidence of pullout, suggesting well-hybridized resin tags. The same features were observed in evaluation of the EDC treated SB (Figure 6d) and SBMP samples after aging. In contrast, the control samples showed pullout and protrusion of resin tags (Figure 6c), both suggesting a lower degree of hybridization than in the corresponding samples that received EDC treatment (Figure 6d).
In evaluation of the control SB specimens subjected to 6 months of aging, the resin adhesive appears to have degraded. It is especially apparent in the remaining tags extending from the fracture surface in Figures 6c and 7a. Due to its larger water sorption and solubility the degradation is more likely for the SB adhesive than SBMP [35]. Although the resin adhesive of the control SBMP specimens did not exhibit signs of degradation (Figure 7b), the collagen fibrils extending to the tags were thinner. In our earlier study concerning the fatigue crack growth resistance of bonded interfaces prepared with SBMP [27], it was unclear if the fracture surfaces revealed degrading matrix or fibrils. However, with these additional results, it is now clearer that the reduction is associated with degrading collagen fibrils at the top of the hybrid layer. Further evaluation by TEM would be recommended as it would provide stronger evidence. While this degradation may have also contributed to the decreased durability of the SB specimens prepared without EDC (Figure 7a), these specimens failed within the region of degrading resin adhesive due to a combination of mechanisms.
The relative contribution of resin and collagen to microtensile bond strength has been explored previously [36]. When the collagen fibrils were well-infiltrated with resin adhesive, the microtensile bond strengths were large (57–62 MPa). When the collagen fibrils were not enveloped with resin, the bonds exhibited significantly lower strength (25–27 MPa). This behavior was interpreted as a mechanical system with parallel loading of resin and collagen fibrils in well-infiltrated bonds (that share stress across the resin and collagen fibrils) and series-loading of resin and collagen fibrils for bonds with constituents that are not well-integrated. Both of these models are relevant to describe the structural behavior after 6-months of hydrolytic aging. In regions of effective resin infiltration, the hybrid layer undergoes parallel loading. But as the collagen fibrils undergo degradation into gelatin in controls without EDC, these regions are replaced by water, allowing the resin to undergo plasticization over time. Under sub-critical mechanical loading the partially degraded collagen fibrils become overstrained and may unravel [36]. Within these regions, the resin adhesive is the only load-bearing structure remaining and undergoes transition from parallel- to localized series-loading. For the interfaces prepared with SB resin adhesive, the fibrils are only weakly reinforced in regions of resin degradation, and the concept of series-loading applies as well. Cyclic loading of these degraded hybrid layers could cause preferential growth of damage within the regions undergoing combined hydrolytic degradation of fibrils and resin, which would accelerate defect growth in the presence of cyclic loading.
Prior studies aimed at bond degradation have utilized microtensile tests and, in general, assessed the changes in bond performance after 12 months storage [e.g. 1,37–40]. In Mazzoni et al. [25] the treatment of etched dentin for 1 minute using a 0.3 M EDC solution resulted in 25 to 35% higher bond strengths after 12 months of water storage. Recall that the rate of cyclic crack growth in the control group was up to 8X higher than in the EDC treated group after 6 months of storage. That is 800%! Clearly the fatigue crack growth resistance of resin-dentin adhesive bonds is more sensitive to degradation than the microtensile strength. That is indicative of the low crack growth resistance of resin adhesives that are not reinforced by collagen fibrils [18]. Collagen fibrils are essential elements to the toughening of the resin adhesive through suppression of the crack opening and the near-tip stress intensity. Degradation of the reinforcing collagen fibrils severely diminishes these mechanisms of toughening.
One limitation of the experimental protocol used for the aging process is that the bonded interfaces were not subjected to cyclic stresses, which contribute to the degree of proteinase activity [41] and mineralization [42,43]. If the collagen fibrils are overstrained by stress, the C-terminal telopeptide of the collagen molecules may be displaced, uncovering the collagenase-susceptible leucine-glycine peptide bond and its attack. Cyclic loading of poorly infiltrated collagen would result in concentrated strain of those fibrils and accelerate degradation with respect to those fibrils in well-infiltrated collagen. According to the differences in resin support evident from the microscopic evaluations, that mechanism would be highly relevant in comparing the durability of the SB and SBMP resin systems. Additional studies addressing synergistic degradation of the interface involving fatigue and MMP activity are underway.
There are some concerns and limitations that are important to highlight. Foremost, the difference in fatigue crack growth resistance was interpreted to result from collagen degradation within the hybrid layer of the control samples by endogenous dentin proteases and the effectiveness of the EDC treatment in preventing this process. Admittedly, that is speculative as the difference in proteinase activity between the EDC and control groups was not quantified. Zymography has been used to show EDC treatments completely inactivated dentinal gelatinases stored in simulated saliva for up to 12 months [26]. Although the similarity in EDC treatments supports that same conclusion, the proteinase activity was not measured and there are other potential contributing mechanisms. EDC is a well-documented collagen crosslinker and has been shown to reduce dentin matrix hydrophilicity [44], which would enhance resin infiltration and the degree of hybridization. In addition, exogenous collagen cross-linking achieved by the EDC treatment [45] would preserve the collagen interfibrillar spaces, further facilitating resin infiltration, hybridization and bond durability.
Lastly, the evaluation consisted of aging and then evaluating the interface durability by cyclic loading afterwards. Accelerated degradation of the dentin collagen by endogeneous proteases is possible through a synergistic response to cyclic loading and oral functions [46–48]. Cyclic stretching of the collagen fibrils within the hybrid layer and consequent water movement within regions of damage may facilitate proteinase activity. Additional studies are needed to assess the effectiveness of EDC treatments in maintaining the fatigue crack growth resistance of resin-dentin bonds over longer time periods.
CONCLUSION
On the basis of the experimental results and analysis, the application of 0.5 M EDC to the demineralized collagen for 1 minute improved the durability of resin-dentin bonds prepared using commercial two-step (SB) and three-step (SBMP) resin adhesive systems. There was no significant difference between the immediate fatigue crack growth resistance of the bonded interfaces prepared with or without the EDC conditioning. The fatigue crack growth resistance of the bonded interfaces prepared using the SB adhesive system was superior to that of SBMP, both immediately after bonding and after 6 months of aging. After 6 months of storage in buffered artificial saliva solution at 37°C the dentin bonds prepared with EDC pre-treatment exhibited significantly greater resistance to the initiation of fatigue crack growth, and up to 8 times lower rate of cyclic extension. The EDC treatment was equally effective in maintaining the durability of the bonded interfaces prepared with both the two- and three-step adhesive systems as evaluated by the fatigue crack growth resistance.
Supplementary Material
Acknowledgments
The authors acknowledge support from the National Institutes of Health (NIDCR R01 DE015306-06 P.I. Pashley) and the National Science Foundation (NSF DMR 1337727 Takacs). The authors also gratefully acknowledge 3M ESPE for their generous donation of bonding supplies and resin composite.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hashimoto M, Ohono H, Kaga M, Endo K, Sano H, Oguchi H. In vivo degradation of resin-dentin bonds in humans over 1 to 3 years. J Dent Res. 2000;79:1385–1391. doi: 10.1177/00220345000790060601. [DOI] [PubMed] [Google Scholar]
- 2.Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater. 2005;21(9):864–81. doi: 10.1016/j.dental.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, et al. Adhesive/dentin interface: the weak link in the composite restoration. Ann Biomed Eng. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tjäderhane L. Dentin bonding: can we make it last? Oper Dent. 2015;40(1):4–18. doi: 10.2341/14-095-BL. [DOI] [PubMed] [Google Scholar]
- 5.Frassetto A, Breschi L, Turco G, Marchesi G, Di Lenarda R, Tay FR, et al. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability-A literature review. Dent Mater. 2016;32(2):e41–53. doi: 10.1016/j.dental.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 6.van Dijken JW, Sunnegardh-Gronberg K, Lindberg A. Clinical long-term retention of etch-and-rinse and self-etch adhesive systems in non-carious cervical lesions. A 13 years evaluation. Dent Mater. 2007;23:1101–7. doi: 10.1016/j.dental.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 7.van Dijken JW. Clinical evaluation of three adhesive esystems in class V non-carious lesions. Dent Mater. 2000;16:285–91. doi: 10.1016/s0109-5641(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 8.van Dijken JW, Pallesen U. Long-term dentin retention of etch-and-rinse and self-etch adhesives and a resin-modified glass ionomer cement in non-carious cervical lesions. Dent Mater. 2008;24(7):915–22. doi: 10.1016/j.dental.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 9.De Munck J, Mine A, Poitevin A, Van Ende A, Cardoso MV, Van Landuyt KL, et al. Meta-analytical review of parameters involved in dentin bonding. J Dent Res. 2012;91(4):351–7. doi: 10.1177/0022034511431251. [DOI] [PubMed] [Google Scholar]
- 10.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83(3):216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 11.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, et al. Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent Mater. 2013;29(1):116–35. doi: 10.1016/j.dental.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, et al. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27(25):4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, et al. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114(2):160–6. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 14.Perdigão J, Reis A, Loguercio AD. Dentin adhesion and MMPs: A comprehensive review. J Esthet Restor Dent. 2013;25(4):219–41. doi: 10.1111/jerd.12016. [DOI] [PubMed] [Google Scholar]
- 15.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24(1):90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, et al. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27(1):1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabatini C, Pashley DH. Mechanisms regulating the degradation of dentin matrices by endogenous dentin proteases and their role in dentin adhesion. A Review. Am J Dent. 2014;27:203–214. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Beitzel D, Mutluay M, Tay FR, Pashley DH, Arola D. On the durability of resin-dentin bonds: Identifying the weakest links. Dent Mater. 2015;31(9):1109–18. doi: 10.1016/j.dental.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedran-Russo AK, Yoo KJ, Ema KC, Pashley DH. Mechanical properties of tannic-acid-treated dentin matrix. J Dent Res. 2009;88:807–11. doi: 10.1177/0022034509342556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Ammar A, Drummond JL, Bedran-Russo AK. The use of collagen cross-linking agents to enhance dentin bond strength. J Biomed Mater Res. 2009;91:419–24. doi: 10.1002/jbm.b.31417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castellan CS, Pereira PN, Grande RHM, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater. 2010;26:968–73. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90:953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedran-Russo AKB, Vidal CMP, Santos PHD, Castellan CS. Long-term effects of carbodiimide on dentin matrix and resin–dentin bonds. J Biomed Mater Res Part B Applied Biomater. 2010;94B:250–5. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tezvergil-Mutluay A, Mutluay MM, Agee KA, Seseogullari-Dirihan R, Hoshika T, Cadenaro M, et al. Carbodiimide cross-linking inactivates soluble and matrix-bound MMPs, in vitro. J Dent Res. 2012;91:192–6. doi: 10.1177/0022034511427705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzoni A, Angeloni V, Apolonio FM, Scotti N, Tjäderhane L, Tezvergil-Mutluay A, et al. Effect of carbodiimide (EDC) on the bond stability of etch-and-rinse adhesive systems. Dent Mater. 2013;29(10):1040–7. doi: 10.1016/j.dental.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Mazzoni A, Apolonio FM, Saboia VP, Santi S, Angeloni V, Checchi V, et al. Carbodiimide inactivation of MMPs and effect on dentin bonding. J Dent Res. 2014;93(3):263–8. doi: 10.1177/0022034513516465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Beitzel D, Mutluay M, Tezvergil-Mutluay A, Tay FR, Pashley DH, et al. Effect of carbodiimide on the fatigue crack growth resistance of resin-dentin bonds. Dent Mater. 2016;32(2):211–22. doi: 10.1016/j.dental.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soappman MJ, Nazari A, Porter JA, Arola D. A comparison of fatigue crack growth in resin composite, dentin and the interface. Dent Mater. 2007;23:608–14. doi: 10.1016/j.dental.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Bajaj D, Sundaram N, Nazari A, Arola D. Age, dehydration and fatigue crack growth in dentin. Biomaterials. 2006;27(11):2507–17. doi: 10.1016/j.biomaterials.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 30.Ivancik J, Majd H, Bajaj D, Romberg E, Arola D. Contributions of aging to the fatigue crack growth resistance of human dentin. Acta Biomater. 2012;8(7):2737–46. doi: 10.1016/j.actbio.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paris PC, Erdogan F. A critical analysis of crack propagation laws. J Basic Eng. 1963;D85:528–34. [Google Scholar]
- 32.Malacarne-Zanon J, Pashley DH, Agee KA, Foulger S, Alvesc MC, Breschi L, et al. Effects of ethanol addition on the water sorption/solubility and percent conversion of comonomers in model dental adhesives. Dent Mater. 2009;25:1275–84. doi: 10.1016/j.dental.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cadenaro M, Breschi L, Rueggeberg FA, Agee K, Di Lenarda R, Carrilho M, et al. Effect of adhesive hydrophilicity and curing time on the permeability of resins bonded to water vs. ethanol-saturated acid-etched dentin. Dent Mater. 2009;25:39–47. doi: 10.1016/j.dental.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bajaj D, Sundaram N, Arola D. An examination of fatigue striations in human dentin: in vitro and in vivo. J Biomed Mater Res B Appl Biomater. 2008;85(1):149–59. doi: 10.1002/jbm.b.30927. [DOI] [PubMed] [Google Scholar]
- 35.Malacarne J, Carvalho RM, de Goes MF, Svizero N, Pashley DH, Tay FR, et al. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22(10):973–80. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Tay FR, Carvalho RM, Yiu CK, King NM, Zhang Y, Agee K, et al. Mechanical disruption of dentin collagen fibrils during resin-dentin bond testing. J Adhes Dent. 2000;2(3):175–92. [PubMed] [Google Scholar]
- 37.Sano H, Yoshikawa T, Pereira PN, Kanemura N, Morigami M, Tagami J, et al. Long-term durability of dentin bonds made with a self-etching primer, in vivo. J Dent Res. 1999;78:909–916. doi: 10.1177/00220345990780041101. [DOI] [PubMed] [Google Scholar]
- 38.Shono Y, Terashita M, Shimada J, Kozono Y, Carvalho RM, Russell CM, et al. Durability of resin-dentin bonds. J Adhes Dent. 1999;1:211–218. [PubMed] [Google Scholar]
- 39.Osorio R, Pisani-Proenca J, Erhardt MC, Osorio E, Aguilera FS, Tay FR, et al. Resistance of ten contemporary adhesives to resin-dentine bond degradation. J Dent. 2008;36(2):163–9. doi: 10.1016/j.jdent.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 40.De Munck J, Van den Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, et al. Inhibition of enzymatic degradation of adhesive-dentin interfaces. J Dent Res. 2009;88(12):1101–6. doi: 10.1177/0022034509346952. [DOI] [PubMed] [Google Scholar]
- 41.Toledano M, Aguilera FS, Yamauti M, Ruiz-Requena ME, Osorio R. In vitro load-induced dentin collagen-stabilization against MMPs degradation. J Mech Behav Biomed Mater. 2013;27:10–8. doi: 10.1016/j.jmbbm.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Toledano M, Aguilera FS, Sauro S, Cabello I, Osorio E, Osorio R. Load cycling enhances bioactivity at the resin-dentin interface. Dent Mater. 2014;30(7):e169–88. doi: 10.1016/j.dental.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Toledano M, Osorio E, Aguilera FS, Sauro S, Cabello I, Osorio R. In vitro mechanical stimulation promoted remineralization at the resin/dentin interface. J Mech Behav Biomed Mater. 2014;30:61–74. doi: 10.1016/j.jmbbm.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Leme AA, Vidal CM, Hassan LS, Bedran-Russo AK. Potential role of surface wettability on the long-term stability of dentin bonds after surface biomodification. J Biomech. 2015;48(10):2067–71. doi: 10.1016/j.jbiomech.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cadenaro M, Fontanive L, Navarra CO, Gobbi P, Mazzoni A, Di Lenarda R, Tay FR, Pashley DH, Breschi L. Effect of carboidiimide on thermal denaturation temperature of dentin collagen. Dent Mater. 2016;32(4):492–8. doi: 10.1016/j.dental.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84(8):741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 47.Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86(6):529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 48.Ricci HA, Sanabe ME, de Souza Costa CA, Pashley DH, Hebling J. Chlorhexidine increases the longevity of in vivo resin-dentin bonds. Eur J Oral Sci. 2010;118(4):411–6. doi: 10.1111/j.1600-0722.2010.00754.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


