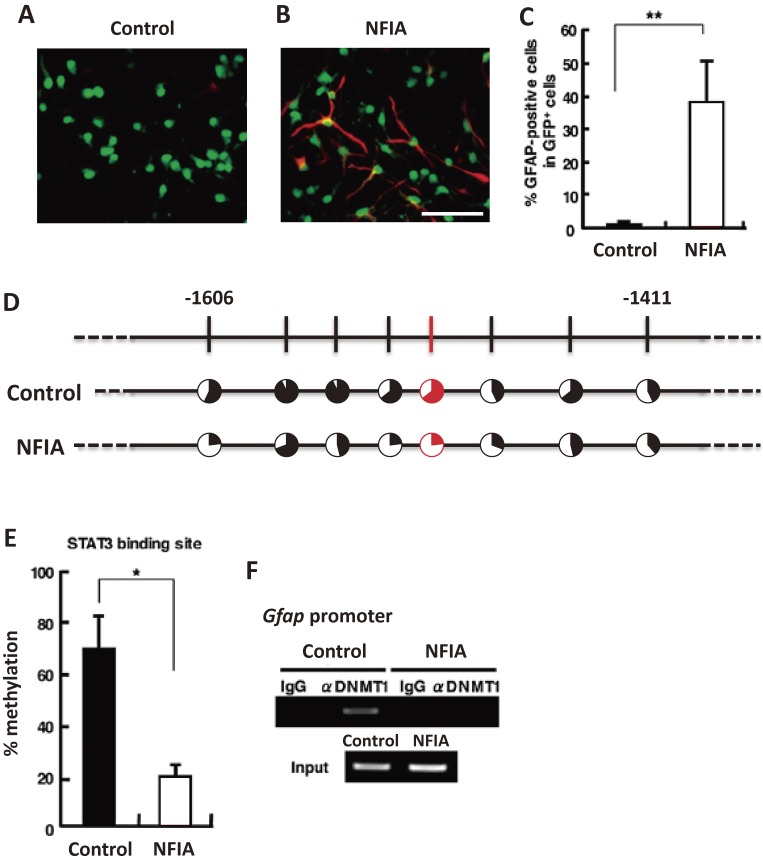
Figure 2.
NFIA potentiates astrocytic differentiation of mgNSCs. A, B. NSCs derived from mouse telencephalons at embryonic day 11.5 (E11.5) were infected with retroviruses engineered to express green fluorescent protein (GFP) alone (A) or GFP together with NFIA (B), cultured for 24 hr in the presence of bFGF, and then stimulated with LIF for a further 3 days to induce astrocytic differentiation. The cells were stained with antibodies against GFP (green) and GFAP (red). Scale bar = 50 µm. C. GFAP-positive astrocytes in GFP control (GFP) and GFP-NFIA-expressing (NFIA) cells were quantified. Data are shown as means ± SD. Statistical significance was examined by Student’s t test (**p < 0.01). D. NSCs derived from E11.5 mouse telencephalons were infected with GFP control (GFP) or GFP-NFIA-expressing (NFIA) retroviruses, and cultured for 4 days with bFGF. After cell sorting based on GFP fluorescence, genomic DNA was extracted, and the methylation status of the Gfap promoter including the STAT3 binding site was examined by bisulfate sequencing. Red indicates STAT3 binding site. Filled portion of circles indicates the percentage of methylation at each CpG site. E. Methylation frequency of the CpG site within the STAT3 binding sequence in the Gfap promoter. Data are shown as means ± SD (n = 3). Statistical significance was examined by Student’s t test (*p < 0.05). F. ChIP assay with a specific antibody for DNMT1 from GFP- and GFP-NFIA-expressing retrovirus-infected NSCs, cultured as in A and B. (Reproduced with modification from Namihira et al., 2009.24))