FIG 4.
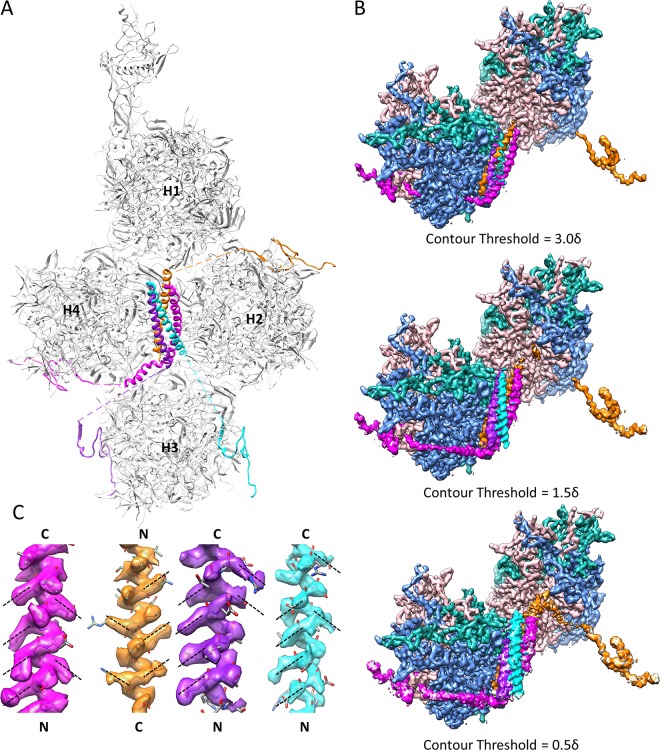
Cement protein IX forms the triskelions and 4-helix bundles on the outer surface of Ad5 capsid. (A) Distribution of four protein IX molecules in each asymmetric unit of Ad5 capsid. The C-terminal regions of four protein IX molecules assemble into a 4-helix bundle, while their N-terminal regions are distributed in four triskelion structures. Quasi-equivalent hexons H1 to H4 are labeled. (B) Densities connecting the N-terminal triskelion domain of protein IX to its C-terminal coiled-coil helix in the 4-helix bundle are discernible by lowering the display threshold. The density map is viewed from the same orientation as that for panel A, with the densities of hexons H2 and H3 hidden for clarity. Only two of the four triskelions and their linker densities to the 4-helix bundle are shown, for clarity. Note that the brown density links the triskelion to a helix from the top of the 4-helix bundle, while the magenta density (and the other two not shown) links the triskelion to a helix from the bottom of the 4-helix bundle. (C) Polarities of the four helices in the 4-helix bundle. Because protein side chains in an α-helix should point slightly toward the N-terminal end of the helix, the polarities of the four helices can be determined unambiguously based on the orientations of side chain densities, as denoted by dashed lines. The four helices were determined to be three parallel and one antiparallel.