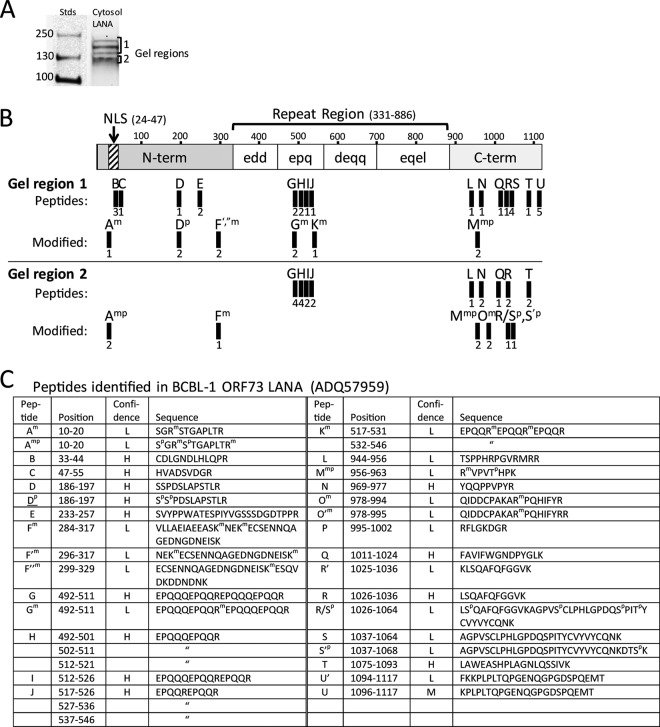
FIG 11.
Full-length LANA isoforms containing the N-terminal NLS motif are identified in the cytosolic fraction of BacK50-induced KSHV-infected Vero cells. (A) The LANA affinity-purified proteins from the cytosol fraction I of the BacK50-induced, KSHV-infected Vero cells (Fig. 10B) were separated by SDS-gel electrophoresis, and gel regions 1 and 2 containing the different LANA isoforms were analyzed separately by mass spectrometry. (B) A schematic of the BCBL-1 LANA structure (1,117 aa) is shown with N-terminal domain containing the major bipartite NLS (58) and the C-terminal domain separated by a large region of different repeated motifs. The trypsin peptides identified by mass spectrometry in the gel regions 1 and 2 are labeled A-U and are mapped onto the structure. Peptide derivatives are labeled with a prime designation (′) and peptides with modifications are indicated with superscript letter (m, methylation; p, phosphorylation). The number of peptide spectrum matches identified in multiple spectrometry analyses is shown below each peptide. No peptides were detected in the “edd,” “deqq,” and “eqel” repeat regions, which lack obvious trypsin cleavage sites. (C) The positions and sequences of the peptides matching the ORF73 LANA sequence in the BCBL-1 strain of KSHV (NCBI accession no. ADQ57959) used for infection are shown, with the positions of modified amino acids. The confidence level of the spectral calls is indicated (H, high; M, medium; L, low).