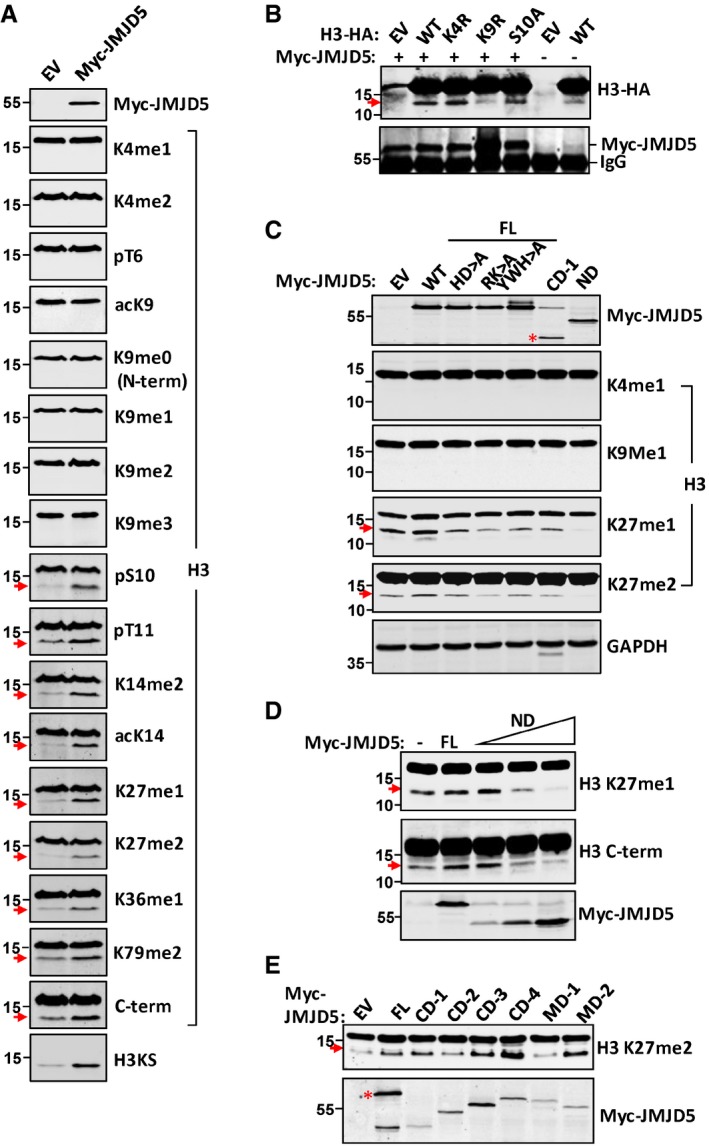
EV or Myc‐JMJD5 was transiently transfected in HeLa cells, and H3 obtained from whole cell lysates was analyzed in Western blot with specific H3 antibodies as indicated.
HA‐tagged WT, K4R, K9R, and S10A mutation form of H3 were cotransfected with or without Myc‐JMJD5 in 293T cells. H3‐HA was detected with anti‐HA antibody in Western blot. Myc‐immunoprecipitates were blotted with anti‐Myc antibody to show Myc‐JMJD5 expression.
HeLa cells were transiently transfected with different domains or mutants of JMJD5 as indicated. Whole cell lysates were subjected to Western blotting analysis with indicated H3 antibodies. Red asterisk indicates corresponding protein expressed by vector.
The dominant negative effect of JMJD5‐ND form on H3 cleavage was examined in HeLa cells with increasing amount of Myc‐JMJD5‐ND. Proteins extracted from these cells were analyzed with indicated H3 antibodies in Western blot.
Full‐length JMJD5 and a series of domain deletion constructs of JMJD5 were transiently transfected in A549 cells. Histone proteins were prepared as above and blotted with anti‐H3K27me2 antibody. JMDJ5 was blotted with anti‐Myc antibody. Red asterisk indicates corresponding protein expressed by vector.
Data information: Red arrow points to cleaved histone H3. Data shown are representative of three independent experiments.