Figure 1.
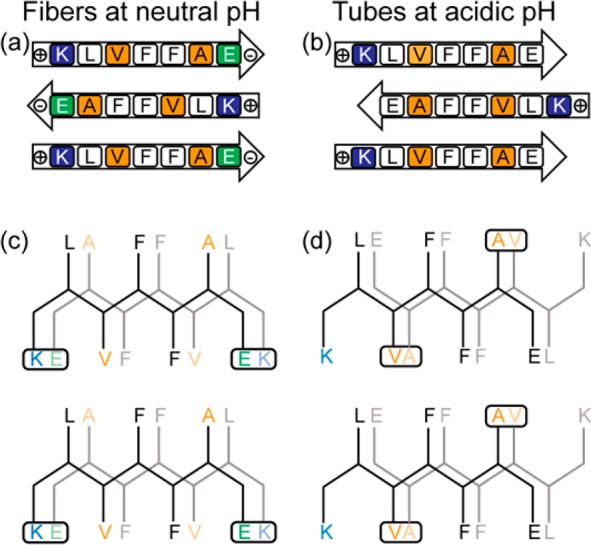
(a, b) Sequence alignment of (a) antiparallel in-register β-sheet and (b) antiparallel out-of-register β-sheet of Aβ(16–22) assemblies. (c, d) Projections down the H-bond axis, with the side chains for the front peptide drawn in black and those for the H-bonded second peptide in gray, highlighting cross-strand side-chain interactions of (c) antiparallel in-register β-sheets and (d) antiparallel out-of-register β-sheets. Positively charged side chains are indicated with blue and negatively charged side chains with green. V and A side chains are highlighted in orange, indicating the preferred packing of the valine side chain with the less bulky alanine side chain.
