FIGURE 3.
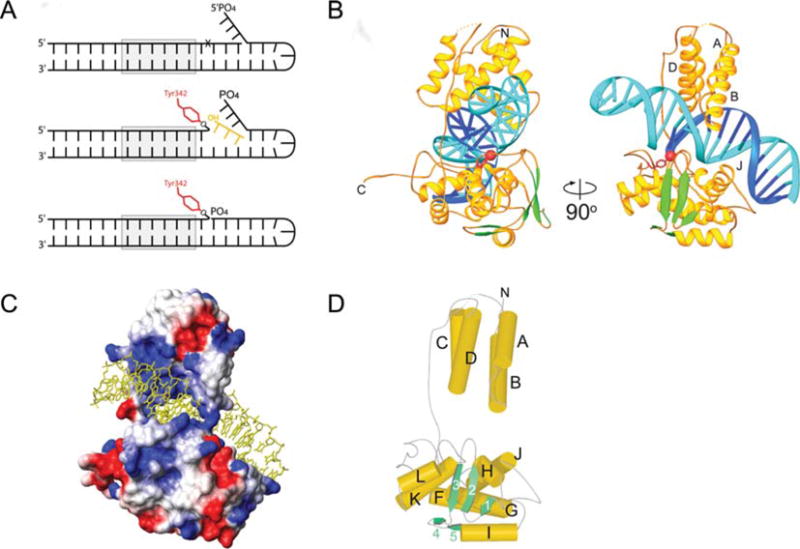
X-ray crystal structure of the Int CTD. (A) With this modified version of previously designed suicide recombination substrates (35, 47) covalently trapped CTD-DNA complexes were stable for weeks. Formation of the phosphotyrosine bond and diffusion of the three base oligonucleotide is followed by annealing of the three base flap to the three nucleotide gap, thus, positioning the 5′-phosphate such that it repels water and shields the phosphotyrosine linkage from hydrolysis. (B) Ribbon diagrams showing the central domain (residues 75 to 160; above the DNA) and the catalytic domain (residues 170 to 356; below the DNA) of λ Int, and their interactions with the major and minor grooves on the opposite sides of the DNA. A long, extended linker (residues I160 to R176) connects these domains. The scissile phosphate that is covalently linked to Y342 is shown as a red sphere. The central domain inserts into the major groove adjacent to the site of DNA cleavage. The catalytic domain makes interactions with the major and minor groove on the opposite side of the DNA, straddling the site of DNA cleavage. (C) The solvent accessible surface of the Int protein is shown, colored according to electrostatic potential. The DNA binding surface is highly positive (blue) and makes numerous interactions with the phosphates of the DNA (cf. Figure 3B). The polypeptide linker between domains joins the central and catalytic domains on one side of the DNA. A salt bridge between the Nζ of K93 and the carbonyl oxygen of S234 bridges between domains on the other side of the DNA, completing the ring-shaped structure that encircles the DNA. (D) The architecture of the λ Int C-75 protein is shown with cylinders and arrows representing helices and β strands, respectively. This view is oriented similarly to that in (A) (right side). The central domain of λ Int lacks helix E, corresponding to the fifth helix of Cre’s N-terminal domain, which is involved in subunit interactions. Reprinted with permission from reference 45. doi:10.1128/microbiolspec.MDNA3-0051-2014.f3
