This cohort study uses the database of the Manchester Centre for Genomic Medicine to analyze lymphocyte DNA of young patients who presented with meningioma or schwannoma before age 25 years.
Key Points
Question
What proportion of apparently solitary schwannomas or meningiomas presenting in children and young people is caused by a heritable predisposing mutation?
Findings
In this study involving 2 cohorts of 177 patients who presented with isolated meningioma or schwannoma before age 25 years, 16 of 42 patients had a predisposing mutation to meningioma and 27 of 135 patients to schwannoma.
Meaning
Genetic testing for young people with a solitary meningioma or schwannoma may be useful for predicting their likelihood of developing additional tumors.
Abstract
Importance
Meningiomas and schwannomas are usually sporadic, isolated tumors occurring in adults older than 60 years and are rare in children and young adults. Multiple schwannomas and/or meningiomas are more frequently associated with a tumor suppressor syndrome and, accordingly, trigger genetic testing, whereas solitary tumors do not. Nevertheless, apparently sporadic tumors in young patients may herald a genetic syndrome.
Objective
To determine the frequency of the known heritable meningioma- or schwannoma-predisposing mutations in children and young adults presenting with a solitary meningioma or schwannoma.
Design, Setting, and Participants
Using the database of the Manchester Centre for Genomic Medicine, this cohort study analyzed lymphocyte DNA from young individuals prospectively referred to the clinic for genetic testing between January 1, 1990, and December 31, 2016, on presentation with a single meningioma (n = 42) or schwannoma (n = 135) before age 25 years. Sequencing data were also examined from an additional 39 patients with neurofibromatosis type 2 who were retrospectively identified as having a solitary tumor before age 25 years. Patients with schwannoma were screened for NF2, SMARCB1, and LZTR1 gene mutations, while patients with meningioma were screened for NF2, SMARCB1, SMARCE1, and SUFU.
Main Outcomes and Measures
The type of underlying genetic mutation, or lack of a predisposing mutation, was associated with the presenting tumor type and subsequent development of additional tumors or other features of known schwannoma- and meningioma-predisposing syndromes.
Results
In 2 cohorts of patients who presented with an isolated meningioma (n = 42; median [range] age, 11 [1-24] years; 22 female) or schwannoma (n = 135; median [range] age, 18 [0.2-24] years; 60 female) before age 25 years, 16 of 42 patients (38%) had a predisposing mutation to meningioma and 27 of 135 patients (20%) to schwannoma, respectively. In the solitary meningioma cohort, 34 of 63 patients (54%) had a constitutional mutation in a known meningioma predisposition gene. Twenty-five of 63 patients (40%) had a constitutional NF2 mutation, and 9 (14%) had a constitutional SMARCE1 mutation. In the cohort of those who developed a solitary schwannoma before age 25 years, 44 of 153 patients (29%) had an identifiable genetic predisposition. Twenty-four patients (55%) with a spinal schwannoma had a constitutional mutation, while only 20 (18%) with a cranial schwannoma had a constitutional predisposition (P < .001). Of 109 cranial schwannomas, 106 (97.2%) were vestibular. Four of 106 people (3.8%) with a cranial schwannoma had an LZTR1 mutation (3 were vestibular schwannomas and 1 was a nonvestibular schwannoma), and 9 (8.5%) had an NF2 mutation.
Conclusions and Relevance
A significant proportion of young people with an apparently sporadic solitary meningioma or schwannoma had a causative predisposition mutation. This finding has important clinical implications because of the risk of additional tumors and the possibility of familial disease. Young patients presenting with a solitary meningioma or schwannoma should be referred for genetic testing.
Introduction
Meningiomas are tumors that develop from the arachnoid layer of the meningeal membrane covering the brain and spinal cord. Schwannomas arise from Schwann cells surrounding nerves. Meningiomas and schwannomas are normally isolated and sporadic but account for almost half of all primary brain and central nervous system tumors in adults. They are rare in children and young adults, with meningiomas accounting for approximately 3% of brain tumors in the pediatric population and schwannomas accounting for approximately 5%. Ninety-four percent of schwannomas are vestibular schwannomas (VSs). The occurrence of multiple meningiomas and/or schwannomas in patients who have not received radiotherapy is unusual and often associated with the tumor suppressor syndrome neurofibromatosis type 2 (NF2) or, less frequently, schwannomatosis. Consequently, the occurrence of multiple meningiomas or schwannomas, unlike solitary tumors, usually triggers genetic evaluation. It is not standard practice to test patients with solitary tumors, but testing in young patients sometimes heralds genetic syndromes with multiple subsequent tumors. Indeed, previous work has shown that 14% of children with isolated meningiomas and 13% with schwannomas will later fulfill the diagnostic criteria for NF2.
Mutation of the NF2 (OMIM 607379) gene is the most common genetic risk factor for meningiomas and schwannomas. Multiple schwannomas and, rarely, multiple meningiomas have also been found in SMARCB1-associated schwannomatosis disease. The SMARCB1 (OMIM 601607) and LZTR1 (OMIM 600574) genes are each causative of approximately 25% of schwannomatosis disease.
A novel syndrome of multiple clear-cell meningiomas, associated with constitutional SMARCE1 (OMIM 603111) mutations, was described in a study. The SMARCB1 and SMARCE1 genes encode subunits of the switch/sucrose nonfermenting chromatin remodelling complexes. Subunits from this complex have been found to be mutated in approximately 20% of all human tumors. The final gene of interest is SUFU (OMIM 607035). In the context of heritable meningioma disease, a germline SUFU mutation has been found in a single large, multigenerational family with multiple meningiomas. Because young patients with NF2 sometimes initially present with a solitary tumor, the known predisposition genes discussed thus far may be responsible for an increased proportion of solitary meningiomas or schwannomas in patients younger than 25 years. Currently, there is no consensus on genetic testing or clinicoradiological surveillance for these patients. Therefore, we sought to determine in patients younger than 25 years the proportion of apparently sporadic meningiomas and schwannomas that are associated with a known constitutional tumor predisposition mutation.
Methods
Manchester NF2 Database
Genetic data were surveyed from a prospectively recorded database held at the Manchester Centre for Genomic Medicine. The database contained details about patients with 1 or more features of NF2 disease ascertained between January 1, 1990, and December 31, 2016, through referral to the department of the Manchester Centre for Genomic Medicine. Ethical approval of the use of anonymized samples from the Manchester Centre for Genomic Medicine archive was obtained from the North West 7–Greater Manchester Central Research Ethics Committee. Written consent was obtained from prospectively recruited individuals.
Materials
We identified lymphocyte DNA from 63 individuals (including 21 retrospectively identified patients with NF2) who presented with a solitary meningioma and 153 (including 18 retrospectively identified patients with NF2) who presented with a solitary schwannoma before age 25 years and without a family history of meningioma or schwannoma. In addition, we collected available tumor DNA of 51 patients for testing. For 7 patients who subsequently developed additional tumors, DNA of more than 1 tumor was available.
Mutation Analysis
All coding exons of NF2, SMARCB1, SMARCE1, and SUFU were sequenced for patients with meningioma by either Sanger sequencing or next-generation sequencing. The NF2, SMARCB1, and LZTR1 genes were analyzed in individuals with a schwannoma, and NF2, SMARCB1, SMARCE1, and SUFU were analyzed in individuals with a meningioma. For Sanger sequencing, exons were amplified by Taq polymerase (GoTaq G2; Promega Corporation), purified using magnetic beads (AxyPrep Mag PCR Clean-Up Kit; Appleton Woods), and sequenced using the Sanger sequencing method (BigDye Terminator v3.1 Cycle Sequencing Kit; ThermoFisher Scientific). Sequencing products were purified using magnetic beads (AxyPrep Mag DyeClean; Appleton Woods) and analyzed on an automated DNA analyzer (ABI 3730xl DNA Analyzer; ThermoFisher Scientific). Since 2014, next-generation sequencing has been used for NF2, SMARCB1, and LZTR1 analysis. For next-generation sequencing, entire coding sequences, including 50 base pairs flanking sequences at either side of each exon, were amplified using long-range polymerase chain reaction followed by next-generation sequencing on a high-throughput benchmark sequencer (Illumina MiSeq; Illumina Inc), giving a minimum coverage of 350 times (mean, 1000 times). Mutation and variant calling was carried out using a custom bioinformatics analysis pipeline. Variant pathogenicity was assessed using in silico mutation prediction algorithms, including PolyPhen2 (Polymorphism Phenotyping, version 2), SIFT (Scale Invariant Feature Transform), Align GVGD, Mutation Taster, Human Splice Finder, NNSPLICE, and GeneSplicer.
Copy Number Analysis
Multiplex ligation–dependent probe amplification of SMARCE1, LZTR1, and SUFU was carried out as described previously. Commercially available multiplex ligation–dependent probe amplification probe sets (P043-NF2 and P258-B1; MRC-Holland) were used to analyze NF2 and SMARCB1, respectively. The products were analyzed on an automated DNA analyzer (ABI Prism 3100 DNA sequencer; ThermoFisher Scientific).
Statistical Analysis
A 2-tailed Fisher exact test was used to calculate statistical significance between tumor groups using a χ2 2 × 2 table. The level of statistical significance was set at 2-sided P < .05.
Results
Frequency of Presentation With Solitary Meningioma or Schwannoma
Interrogation of the Manchester database identified 319 individuals with de novo NF2 disease presenting with a tumor diagnosis before age 25 years. Twenty-nine of these (9.1%; median [range] age, 9 [1-21] years; 10 female) had symptomatic presentations with an initial, apparently sporadic meningioma, 16 (5.0%; median [range] age, 12 [6-20] years; 5 female) had an apparently isolated spinal schwannoma, and 24 (7.5%; median [range] age, 9 [2-18] years; 14 female) presented with an intracutaneous schwannoma prior to the diagnosis of other tumors (eTable 1 in the Supplement). In addition, 19 (6.0%; median [range] age, 18 [1-22] years; 11 female) presented with an isolated cranial schwannoma (eTable 2 in the Supplement). As such, 88 of the 319 patients (27.6%) with de novo NF2 disease before age 25 years presented with a solitary tumor. Patients with intradermal or cutaneous schwannomas are known already to have an extremely high chance of the schwannoma being caused by NF2, and the presence of intradermal or cutaneous schwannoma is an exclusion criterion for schwannomatosis. In addition, this lesion has not been referred to our diagnostic service as a stand-alone lesion. Therefore, we excluded patients who presented with an intracutaneous plaque schwannoma or plexiform schwannoma as the initial tumor from the remainder of the study.
Frequency of Heritable Genetic Cause
Next, we searched the entire Manchester database for individuals referred to the clinical genetics clinic with an isolated schwannoma or meningioma occurring before age 25 years but without a family history of schwannoma or meningioma disease; we did not limit our search to those with a clinical diagnosis of NF2 prior to genetic testing. From this cohort, we identified 65 individuals with an isolated meningioma, and the DNA of 63 of them (96.9%) was available for genetic testing. There were also 188 individuals with an isolated schwannoma, and the lymphocyte DNA of 153 of them (81.4%) was available. We determined the frequency of mutations in the known predisposition genes by analyzing NF2, SMARCB1, SMARCE1, and SUFU in individuals with an isolated meningioma and NF2, SMARCB1, and LZTR1 in individuals with an isolated schwannoma. Only mutations previously associated with disease or predicted to be damaging by multiple in silico algorithms were included in the study. Known polymorphisms, synonymous alterations, and variants of unknown significance were excluded from the results.
Frequency of Meningioma Predisposition
In the solitary meningioma cohort, 34 of 63 patients (54.0%) had a constitutional mutation in a known meningioma predisposition gene (Table 1). Twenty-five of 63 patients (39.7%) had a constitutional NF2 mutation, and 9 (14.3%) had a constitutional SMARCE1 mutation. We found no constitutional mutations in SMARCB1 or SUFU. We found a higher likelihood of predisposition in those with a spinal meningioma than in those with a cranial meningioma (18 of 22 patients [81.8%] vs 16 of 41 [39.0%]; P = .001).
Table 1. Constitutional Meningioma Predisposition Mutations in Individuals Presenting With a Solitary Meningioma Before Age 25 Years.
| Characteristic | No./Total No. (%) [Mosaic Mutation] | ||
|---|---|---|---|
| NF2 Mutation | SMARCE1 Mutation | No Constitutional Mutation Identified |
|
| Including Retrospective NF2 | |||
| Total | 25/63 (40) [5] | 9/63 (14) | 29/63 (46) |
| Age at onset | |||
| 1-15 y | 19/47 (40) [4] | 5/47 (11) | 23/47 (49) |
| 16-24 y | 6/16 (38) [1] | 4/16 (25) | 6/16 (38) |
| Type of meningioma | |||
| Cranial | 11/41 (27) [1] | 5/41 (12) | 25/41 (61) |
| Spinal | 14/22 (64) [4] | 4/22 (18) | 4/22 (18) |
| Excluding Retrospective NF2 | |||
| Total | 7/42 (17) [1] | 9/42 (21) | 26/42 (62) |
| Age at onset | |||
| 1-15 y | 7/32 (22) [1] | 5/32 (16) | 20/32 (63) |
| 16-24 y | 0/10 (0) | 4/10 (40) | 6/10 (60) |
| Type of meningioma | |||
| Cranial | 7/36 (19) [1] | 5/36 (14) | 24/36 (67) |
| Spinal | 0/6 (0) | 4/6 (67) | 2/6 (33) |
Abbreviation: NF2, neurofibromatosis type 2.
Data for this cohort included retrospectively identified cases of NF2 disease who initially presented with a single meningioma before age 25 years but were not tested until they developed other features of NF2. Analysis of the cohort, including only patients with DNA prospectively isolated at the time of presentation (n = 42) (median [range] age, 11 [1-24] years; 22 female), showed that 16 of 42 patients (38.1%) had a constitutional mutation. Of these 42 patients, 7 (16.7%) had an NF2 mutation and 9 (21.4%) had a SMARCE1 mutation (Table 1).
Of those younger than 16 years (n = 32), 7 (21.9%) had an NF2 mutation and 5 (15.6%) had a SMARCE1 mutation. In those aged 16 to 24 years (N = 10), no one had an NF2 mutation but 4 (40.0%) had a SMARCE1 mutation. Identification of a SMARCE1 mutation in 1 proband led to the genetic testing of 2 relatives, who were both found to carry the same mutation (not included in the statistics). Reflexive imaging of these individuals led to the identification of an asymptomatic cranial and a spinal meningioma in the mother and the diagnosis of a spinal meningioma requiring surgery in the brother. Another proband with an identified SMARCE1 mutation has subsequently shown tumor recurrence. Reflexive testing of the father found that he carries the same mutation but is currently unaffected at age 38 years, with normal findings on brain magnetic resonance imaging. Spinal magnetic resonance imaging is planned but had not been carried out at the time of this report.
Frequency of Schwannoma Predisposition
Of the 153 individuals in the cohort who developed a solitary schwannoma before age 25 years, 44 (28.8%) had an identifiable genetic predisposition (Table 2). Of 44 patients with a spinal schwannoma, 24 (54.5%) had a constitutional mutation, whereas only 20 of 109 with a cranial schwannoma (18.3%) had a constitutional predisposition (odds ratio, 0.1873; 95% CI, 0.0870-0.4030; P < .001). Of 109 cranial schwannomas, 106 (97.2%) were vestibular. Four of 106 people (3.8%) with a cranial schwannoma had an LZTR1 mutation (3 were VS and 1 was non-VS), and 9 (8.5%) had an NF2 mutation. We found no SMARCB1 mutations in this cohort.
Table 2. Constitutional Schwannoma Predisposition Mutations in Individuals Presenting With a Solitary Schwannoma Before Age 25 Years.
| Characteristic | No./Total No. (%) [Mosaic Mutation] | |||
|---|---|---|---|---|
| NF2 Mutation | LZTR1 Mutation |
SMARCB1
Mutation |
No Constitutional Mutation Identified |
|
| Including Retrospective NF2 | ||||
| Total | 28/153 (18) [13] | 10/153 (6) | 6/153 (4) | 109/153 (71) |
| Age at onset | ||||
| 1-15 y | 14/56 (25) [4] | 5/56 (9) | 1/56 (2) | 36/56 (64) |
| 16-24 y | 14/97 (14) [9] | 5/97 (5) | 5/97 (5) | 73/97 (75) |
| Type of schwannoma | ||||
| Cranial | 16/109 (15) [9] | 4/109 (4) | 0/109 (0) | 90/109 (81) |
| Spinal | 12/44 (27) [4] | 6/44 (14) | 6/44 (14) | 20/44 (45) |
| Excluding Retrospective NF2 | ||||
| Total | 11/135 (8) [4] | 10/135 (7) | 6/135 (4) | 108/135 (80) |
| Age at onset | ||||
| 1-15 y | 7/49 (14) [2] | 5/49 (10) | 1/49 (2) | 36/49 (73) |
| 16-24 y | 4/86 (5) [2] | 5/86 (6) | 5/86 (6) | 70/86 (84) |
| Type of schwannoma | ||||
| Cranial | 6/98 (6) [2] | 4/98 (4) | 0/98 (0) | 88/98 (90) |
| Spinal | 5/37 (14) [2] | 6/37 (16) | 6/37 (16) | 20/37 (54) |
Abbreviation: NF2, neurofibromatosis type 2.
A higher proportion of NF2 mutations were identified in those younger than 16 years compared with those aged 16 to 24 years. A higher proportion of schwannomatosis mutations was found in the 16- to 24-year age group mainly because of an increase in SMARCB1-associated schwannomatosis.
Removing the retrospectively identified patients with NF2 disease from the schwannoma cohort left a prospectively determined cohort of 135 individuals (median [range] age, 18 [0.2-24] years; 60 female) (Table 2) with a similar overall pattern of mutations.
Mosaicism
Eleven cases of low-level mosaicism (5 meningiomas and 6 schwannomas) were identified on blood analysis. In some instances, no mutation was detected in blood, but the individual had subsequently developed more than 1 tumor. In these cases in which DNA was available from 2 of these 7 tumors (all initial tumors were schwannomas), both tumors were screened to determine whether they were the result of mosaicism, which was assumed when an identical mutation was found in each tumor. All of the identified cases of mosaicism were caused by NF2 mutations (Tables 1 and 2). No mosaic mutations were found for SMARCE1, SMARCB1, and LZTR1, although the availability of tumor DNA for analysis in these groups was reduced. Detection of mosaicism by tumor analysis was not possible in most cases because no second tumor DNA was available for analysis.
Discussion
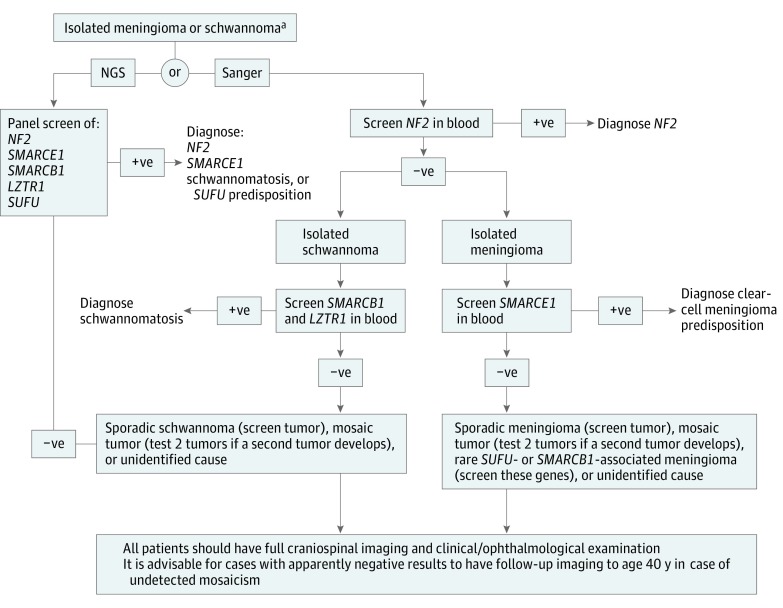
We have discovered a high frequency of constitutional genetic predisposition in people younger than 25 years who presented with an apparently isolated schwannoma or meningioma. In the unbiased cohort that does not contain retrospectively selected individuals with NF2 disease, 16 of 42 solitary meningiomas (38.1%) and 27 of 135 solitary schwannomas (20.0%) in children and young adults were associated with known genetic predisposition mutations. These data build on previous epidemiological work that suggested a risk of disease predisposition in apparently sporadic childhood tumors. Now that we have confirmed a high likelihood of constitutional tumor suppressor mutations, there are immediate clinical implications (Figure) for this category of patients and their families because they are at risk of developing further tumors.
Figure. Genetic Testing Recommendations for Individuals Presenting With Isolated Meningioma or Schwannoma at a Young Age.
NF2 indicates neurofibromatosis type 2; NGS, next-generation sequencing; +ve, pathogenic mutation identified on sequencing; and –ve, pathogenic mutation not identified on sequencing.
aIf the schwannoma is intracutaneous, only need to test NF2.
We included 2 cohorts of patients in our analysis: those referred for genetic evaluation at the time of solitary tumor diagnosis purely because of their young age at presentation (no other clinical or imaging features of tumor suppressor syndromes) and those referred for genetic testing at the time of developing other clinical features of NF2 or meeting NF2 criteria, having initially presented with a solitary tumor at a young age. The former cohort is an unbiased group, and these data were used to determine the frequency of known, causative constitutional mutations in young patients with solitary tumors. Although in many cases we performed full craniospinal imaging to ensure the solitary nature of the index tumor, we do not have information on whether this imaging was true for all patients, and a caveat is that some may have had brain or spine imaging alone according to varying institutional protocols at the time. The most common cause of genetic predisposition to meningiomas and schwannomas is NF2, and the NF2 cohort detailed in this article sheds further light on the presentation and natural history of this disease when it manifests in those younger than 25 years (eTable 1 and eTable 2 in the Supplement).
These 2 cohorts are complimentary but are presented with and without the retrospective cases because of the large size of the NF2 service of the genetics clinic, which could result in bias and overestimation of NF2 frequency. Therefore, we caution against extrapolation of these NF2 data to the general population, which is likely to have occurred in an older study of 53 pediatric meningiomas in which NF2 cases were mixed with apparently isolated cases, giving an overall 40% figure for NF2. Exclusion of those with known NF2 would have greatly reduced this figure for isolated tumors. Nonetheless, retrospective NF2 cases were useful as a reference for estimating the frequency of mosaicism for NF2 given that many of the unbiased cohort did not have 2 tumors during the study period to test for mosaicism. Therefore, it is possible that those patients who were still young at the last follow-up could have mosaic NF2 and thus would be likely to present with additional tumors. The exclusion of the retrospectively identified patients with NF2, along with the exclusion of retrospectively collected patients with meningioma specifically referred to the clinic following the discovery of the association of SMARCE1 mutations with clear-cell meningioma, makes the results from the unbiased cohort more generalizable to other patient populations. To our knowledge, no referral center bias occurred in the prospective cohort presented here, but it will be important to see whether these data are replicated by other groups.
In the meningioma cohort, including retrospective patients with NF2, 5 of 25 NF2 gene mutations (20.0%) found in young patients with a solitary meningioma were mosaic. This number was lower in the cohort without retrospectively identified patients with NF2, with only 1 mosaic individual; however, the overall incidence of mosaicism could be higher because 27 of 29 patients (93.1%) with meningioma who had no identified constitutional mutation did not have 2 tumors available for genetic testing. In patients with schwannoma, approximately half of the NF2 mutations identified were mosaic. This proportion was higher in cranial schwannomas than in spinal schwannomas, which may reflect the probability that cranial imaging was not carried out at the time of diagnosis to exclude the presence of VS in many cases. Overall, 85% of patients with NF2 presenting with VS disease presented bilaterally. In the total schwannoma group without retrospective patients with NF2, this figure could be an underestimate because 107 of 108 patients (99.1%) with no identified mutation did not have 2 tumors available for mosaicism testing. Thus, the rates of tumor suppressor mutations may actually be higher than that shown because of further unidentified mosaic cases. Given that the mosaicism rate is around 30% for patients presenting with de novo classic NF2 with bilateral VS, a high degree of suspicion of mosaic disease should still exist even after negative blood testing.
In the unbiased cohort of 42 patients presenting with a solitary meningioma, 16 cases (38.1%) were associated with an underlying predisposing mutation. We found a high incidence of SMARCE1-associated meningiomas, which were all clear-cell meningiomas. A review of meningiomas occurring in the 0- to 19-year age group found that clear-cell meningiomas comprised 0.3% of meningiomas in this age group. We found that the incidence of SMARCE1 mutations, known to occur in clear-cell meningiomas, was much higher than this percentage despite excluding retrospectively identified clear-cell referrals. We found an overrepresentation of clear-cell meningiomas occurring in young people.
Our results from the cranial schwannoma cohort were similar to previous data suggesting that constitutional LZTR1 mutations are as common as nonmosaic NF2 mutations in patients fulfilling the NF2 criteria with a unilateral VS and other schwannomas. In the current data set, however, 1 of the constitutional LZTR1 mutations was found in a patient with a solitary facial nerve schwannoma rather than a VS. Only 19 of 106 patients (17.9%) with VS had a constitutional mutation. Sixteen of these were NF2 mutations and 3 were LZTR1 mutations. This group had the lowest incidence of predisposition. It has been shown that, in NF2 disease, most VSs present bilaterally; when unilateral VSs occur in young people, there is a reduced association with NF2 disease.
We have shown that young people presenting with a solitary schwannoma or meningioma have a high likelihood of carrying a constitutional predisposition mutation. This finding is especially true of spinal tumors and tumors occurring in the youngest age groups (1-15 years). The most common cause of predisposition is NF2 disease, whereas the risk from SMARCE1 meningiomas is increased in young adults (16-24 years). Presence of the LZTR1 gene shows an increased risk of schwannomas in the younger age group (1-15 years), whereas SMARCB1-associated schwannomas develop later during adolescence. Solitary VS had the lowest risk of being caused by a constitutional mutation.
Limitations
There are some limitations to this study. Not all patients were tested at the time of, or soon after, the diagnosis of their original, apparently sporadic tumor. Not all patients had full clinical assessment for NF2 with craniospinal imaging and a detailed cutaneous examination. With these extra examinations at baseline, a small proportion of patients with a truly sporadic tumor may possibly have NF2.
Conclusions
Results of this study highlight the need for clinical assessment and genetic testing in young people with an isolated meningioma or schwannoma. We recommend that young patients presenting with an isolated meningioma or schwannoma, including those with intracutaneous schwannomas, obtain a full assessment to exclude additional tumors. This assessment should include full craniospinal magnetic resonance imaging with an NF2 protocol that includes 1-mm imaging-section thickness through the internal auditory meatus and after the use of contrast imaging. A full dermatologic examination for NF2 plaques and an ophthalmologic examination for retinal hamartoma and lens opacity are also advised. Our recommended genetic testing strategies are shown in the Figure. We did not find any SMARCB1- or SUFU-associated cases of meningiomas. However, SMARCB1 and SUFU are known to be rare causes of meningiomas in individuals without an NF2 or SMARCE1 mutation and may be present at low levels in a larger cohort. Thorough genetic testing will lead to appropriate screening of relatives and subsequent imaging for early detection of tumors in relatives. Furthermore, even in those without a proven constitutional mutation, some form of follow-up imaging is advisable until approximately age 40 years because many patients with NF2 present with mosaic disease not identifiable in the blood.
eTable 1. NF2 Patients Diagnosed <25 With an Apparently Isolated Meningioma or Non-Vestibular Schwannoma and Subsequent Tumour Development
eTable 2. Characteristics of NF2 Patients Diagnosed <25 With an Apparently Isolated Vestibular Schwannoma and Subsequent Tumour Development
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16(suppl 4):iv1-iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christiaans I, Kenter SB, Brink HC, et al. . Germline SMARCB1 mutation and somatic NF2 mutations in familial multiple meningiomas. J Med Genet. 2011;48(2):93-97. [DOI] [PubMed] [Google Scholar]
- 3.Evans DG, Birch JM, Ramsden RT. Paediatric presentation of type 2 neurofibromatosis. Arch Dis Child. 1999;81(6):496-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans DG, Huson SM, Donnai D, et al. . A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84(304):603-618. [PubMed] [Google Scholar]
- 5.Smith MJ, O’Sullivan J, Bhaskar SS, et al. . Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas. Nat Genet. 2013;45(3):295-298. [DOI] [PubMed] [Google Scholar]
- 6.Smith MJ, Wallace AJ, Bennett C, et al. . Germline SMARCE1 mutations predispose to both spinal and cranial clear cell meningiomas. J Pathol. 2014;234(4):436-440. [DOI] [PubMed] [Google Scholar]
- 7.Kadoch C, Hargreaves DC, Hodges C, et al. . Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet. 2013;45(6):592-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aavikko M, Li SP, Saarinen S, et al. . Loss of SUFU function in familial multiple meningioma. Am J Hum Genet. 2012;91(3):520-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith MJ, Beetz C, Williams SG, et al. . Germline mutations in SUFU cause Gorlin syndrome–associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol. 2014;32(36):4155-4161. [DOI] [PubMed] [Google Scholar]
- 10.Evans DG. Neurofibromatosis type 2 (NF2): a clinical and molecular review. Orphanet J Rare Dis. 2009;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacCollin M, Chiocca EA, Evans DG, et al. . Diagnostic criteria for schwannomatosis. Neurology. 2005;64(11):1838-1845. [DOI] [PubMed] [Google Scholar]
- 12.Perry A, Giannini C, Raghavan R, et al. . Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: a clinicopathologic study of 53 cases. J Neuropathol Exp Neurol. 2001;60(10):994-1003. [DOI] [PubMed] [Google Scholar]
- 13.Baser ME, Friedman JM, Wallace AJ, Ramsden RT, Joe H, Evans DG. Evaluation of clinical diagnostic criteria for neurofibromatosis 2. Neurology. 2002;59(11):1759-1765. [DOI] [PubMed] [Google Scholar]
- 14.Evans DG, Ramsden RT, Shenton A, et al. . Mosaicism in neurofibromatosis type 2: an update of risk based on uni/bilaterality of vestibular schwannoma at presentation and sensitive mutation analysis including multiple ligation-dependent probe amplification. J Med Genet. 2007;44(7):424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caroli E, Russillo M, Ferrante L. Intracranial meningiomas in children: report of 27 new cases and critical analysis of 440 cases reported in the literature. J Child Neurol. 2006;21(1):31-36. [DOI] [PubMed] [Google Scholar]
- 16.Smith MJ, Bowers NL, Bulman M, et al. . Revisiting neurofibromatosis type 2 diagnostic criteria to exclude LZTR1-related schwannomatosis. Neurology. 2017;88(1):87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans DG, Ramsden RT, Gokhale C, Bowers N, Huson SM, Wallace A. Should NF2 mutation screening be undertaken in patients with an apparently isolated vestibular schwannoma? Clin Genet. 2007;71(4):354-358. [DOI] [PubMed] [Google Scholar]
- 18.Evans DG, Raymond FL, Barwell JG, Halliday D. Genetic testing and screening of individuals at risk of NF2. Clin Genet. 2012;82(5):416-424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. NF2 Patients Diagnosed <25 With an Apparently Isolated Meningioma or Non-Vestibular Schwannoma and Subsequent Tumour Development
eTable 2. Characteristics of NF2 Patients Diagnosed <25 With an Apparently Isolated Vestibular Schwannoma and Subsequent Tumour Development