Key Points
Question
Can a novel sustained release buprenorphine weekly injectable formulation (CAM2038) produce robust opioid blockade and opioid withdrawal suppression?
Findings
This randomized clinical trial enrolled individuals with opioid use disorder but not seeking treatment and examined the response to hydromorphone before and after administration of CAM2038 at 2 doses. Both CAM2038 doses produced robust opioid blockade on the primary outcome measure of subjective response for liking of hydromorphone and immediate and sustained withdrawal suppression.
Meaning
Weekly injectable CAM2038 shows promise as a potential treatment for individuals with opioid use disorder.
Abstract
Importance
Buprenorphine is an efficacious, widely used treatment for opioid use disorder (OUD). Daily oral transmucosal formulations can be associated with misuse, diversion, and nonadherence; these limitations may be obviated by a sustained release formulation.
Objective
To evaluate the ability of a novel, weekly, subcutaneous buprenorphine depot formulation, CAM2038, to block euphorigenic opioid effects and suppress opioid withdrawal in non–treatment-seeking individuals with OUD.
Design, Setting, and Participants
This multisite, double-blind, randomized within-patient study was conducted at 3 controlled inpatient research facilities. It involved 47 adults with DSM-V moderate-to-severe OUD. The study was conducted from October 12, 2015 (first patient enrolled), to April 21, 2016 (last patient visit).
Interventions
A total of five 3-day test sessions evaluated the response to hydromorphone (0, 6, and 18 mg intramuscular in random order; 1 dose/session/day). After the first 3-day session (ie, qualification phase), participants were randomized to either CAM2038 weekly at 24 mg (n = 22) or 32 mg (n = 25); the assigned CAM2038 dose was given twice, 1 week apart (day 0 and 7). Four sets of sessions were conducted after randomization (days 1-3, 4-6, 8-10, and 11-13).
Main Outcomes and Measures
The primary end point was maximum rating on the visual analog scale for drug liking. Secondary end points included other visual analog scale (eg, high and desire to use), opioid withdrawal scales, and physiological and pharmacokinetic outcomes.
Results
A total of 46 of 47 randomized participants (mean [SD] age, 35.5 [9] years; 76% male [n = 35]) completed the study. Both weekly CAM2038 doses produced immediate and sustained blockade of hydromorphone effects (liking maximum effect, CAM2038, 24 mg: effect size, 0.813; P < .001, and CAM2038, 32 mg: effect size, 0.753; P < .001) and suppression of withdrawal (Clinical Opiate Withdrawal Scale, CAM2038, 24 mg: effect size, 0.617; P < .001, and CAM2038, 32 mg: effect size, 0.751; P < .001). CAM2038 produces a rapid initial rise of buprenorphine in plasma with maximum concentration around 24 hours, with an apparent half-life of 4 to 5 days and approximately 50% accumulation of trough concentration from first to second dose (trough concentration = 0.822 and 1.23 ng/mL for weeks 1 and 2, respectively, with 24 mg; trough concentration = 0.993 and 1.47 ng/mL for weeks 1 and 2, respectively, with 32 mg).
Conclusions and Relevance
CAM2038 weekly, 24 and 32 mg, was safely tolerated and produced immediate and sustained opioid blockade and withdrawal suppression. The results support the use of this depot formulation for treatment initiation and stabilization of patients with OUD, with the further benefit of obviating the risk for misuse and diversion of daily buprenorphine while retaining its therapeutic benefits.
Trial Registration
Clinicaltrials.gov Identifier: NCT02611752
This randomized clinical trial evaluates the ability of a novel subcutaneous buprenorphine depot formulation (CAM2038) to block euphorigenic opioid effects and suppress opioid withdrawal in nontreatment-seeking individuals with opioid use disorder.
Introduction
According to the World Drug Report, approximately 33 million individuals globally use opioids for nonmedical purposes. Opioids top the list of drugs that cause the greatest burden of disease and drug-related deaths worldwide. A primary goal of treatment for opioid use disorder (OUD) is to help patients reduce or eliminate illicit opioid use, as this facilitates achievement of other important goals, including improved health and psychosocial functioning. Clinical trials have shown that buprenorphine is an efficacious treatment for OUD; it reduces illicit opioid use, retains patients in treatment, and reduces mortality. Buprenorphine offers a key advantage over methadone with its superior safety profile including lower risks for respiratory depression and overdose. Clinical laboratory studies have shown that sublingual (SL) buprenorphine produces a dose-dependent reduction in opioid withdrawal and attenuates opioid craving and the euphorigenic effects of exogenously administered opioids, 3 critical mechanisms that reduce ongoing illicit opioid use.
Unfortunately, buprenorphine itself has abuse liability, is diverted, and has become the primary opioid of abuse in some countries. Sublingual formulations of buprenorphine can be injected or snorted to enhance euphoric effects. Unintentional overdose with buprenorphine has been reported, leading to toxicity and fatality in children and those who coingest buprenorphine with benzodiazepines or alcohol. To address these risks, sustained-release subcutaneous buprenorphine formulations (CAM2038; weekly and monthly depots) were developed with FluidCrystal injection depot technology. CAM2038 is administered using a prefilled syringe with a needlestick safety device requiring no mixing or temperature adjustment before injection. Once injected, it spontaneously transforms from a low viscous solution to a highly viscous liquid crystalline gel that encapsulates buprenorphine and releases it at a steady rate as the depot biodegrades. CAM2038 is developed in different fixed-dose compositions producing plasma concentrations within the SL therapeutic range.
This phase 2 study examined the efficacy of once-weekly CAM2038 at 2 doses, 24 mg and 32 mg (developed to produce plasma buprenorphine exposures within the range [ie, maximum concentration–trough concentration] of SL buprenorphine, approximately 16 mg and 24 mg SL, respectively), to block the subjective drug liking response to acute intramuscular hydromorphone and suppress opioid withdrawal in an opioid-dependent, non–treatment-seeking population. In addition, the pharmacokinetic and safety profiles of CAM2038 were examined. It was hypothesized that both CAM2038 doses would produce clinically relevant opioid blockade based on pharmacokinetic modeling; thus, no between-dose CAM2038 comparisons were planned.
Methods
Study Population
Healthy adult volunteers (18-55 years) with moderate-to-severe OUD (DSM-V) and physical dependence on short-acting opioids (nonmedical use ≥21 of 30 days preceding enrollment) were recruited. Opioid use/physical dependence was verified by self-report, urine drug screens, and/or naloxone challenge. Participants were recruited through local advertisements or existing site databases and compensated for participating. Individuals seeking OUD treatment were excluded and provided referrals. The study was conducted from October 12, 2015 (first patient enrolled), to April 21, 2016 (last patient visit). The protocol is available in Supplement 1.
Medical history and physical examination, an electrocardiogram, blood and urine tests, and a psychiatric interview determined health status. Exclusion criteria included physiological dependence on drugs other than opioids requiring medical treatment; pregnant or breastfeeding; body mass index (calculated as weight in kilograms divided by height in meters squared) greater than 30; chronic pain requiring opioid therapy; AIDS; history/current evidence of suicidal ideation (Columbia-Suicide Severity Rating Scale, grade 4/5); required use of cytochrome P450 3A4 inhibitors/inducers or last 30-day use of monoamine oxidase inhibitors or investigational drugs; clinically significant abnormality based on history/examination/testing; aspartate aminotransferase and/or alanine aminotransferase levels greater than 3 times the upper limit of normal; total bilirubin or creatinine levels greater than 1.5 times the upper limit of normal; or history or presence of any clinically significant illness (eg, depression and diabetes). Outpatient screening was completed within 4 weeks of admission.
Study Setting, Design, and Initial Stabilization
This phase 2 approximately 3-week inpatient randomized, double-blind, within-patient trial was conducted under investigational new drug application No. 114082. Three sites participated (2 academic and 1 commercial with 24-hour nursing coverage), and each received institutional review board approval from the respective site. Participants provided written informed consent before undergoing any study-related procedure. After admission, participants were stabilized with oral morphine, 30 mg 4 times daily (unblinded), for 3 to 7 days before testing, allowing time for washout of illicit opioid use. Hydroxyzine, bismuth subsalicylate, clonidine, zolpidem, acetaminophen, ibuprofen, promethazine, alumina, magnesia, and simethicone were available as needed for mitigating withdrawal symptoms, but not during test session days from midnight until session completion.
Qualification Phase (Days −3 to −1)
Two morphine doses were withheld before each qualification session (the evening before and morning of) to preclude morphine carryover effects. A single intramuscular injection (1.8 mL) of double-blind hydromorphone (0, 6, or 18 mg; randomized order) was given on 3 consecutive days. Study outcomes were collected before and for approximately 5 hours after dosing. Qualification criteria for randomization were prespecified for “at this moment drug liking” maximum visual analog scale (VAS) score (maximum score; maximum effect [Emax]) as follows: 40 to 60 mm for 0 mg; ≥15-mm difference between 0 and 6 mg; ≥20 mm between 0 and 18 mg; and ≥55 mm for 6 mg and ≥60 mm for 18 mg. Morphine dosing ceased after the final qualification test session.
Randomization and CAM2038 Treatment Phase
Those qualified were randomized in a 1:1 ratio to 24- or 32-mg CAM2038 stratified by sex. Participants received 2 once-weekly CAM2038 injections (days 0 and 7). To preclude buprenorphine-precipitated withdrawal, participants were required to exhibit mild withdrawal with a Clinical Opiate Withdrawal Scale (COWS) score of 8 or more before the first CAM2038 injection; the COWS was repeated until the threshold was reached. The COWS consists of 11 opioid-withdrawal signs rated by a trained observer on a scale from 0 to 4 (total scale range, 0-44, where 5 to 12 represents mild symptoms). The timing of subsequent challenge sessions was adjusted to map onto 24-hour intervals after CAM2038 dosing.
Four sets of 3 hydromorphone test sessions, identical to the qualification sessions, were conducted on days 1 to 3, days 4 to 6, days 8 to 10, and days 11 to 13 to evaluate the time course of response to CAM2038 injection. Discharge was on day 14, with a follow-up telephone call at 7 days after discharge.
Drugs and Dosing Procedures
Morphine sulfate tablets (30 mg; Roxane Laboratories), hydromorphone (10 mg/mL; Hospira Inc), and 0.45% saline (Hospira Inc) were purchased as single-dose vials. Intramuscular hydromorphone injections rotated between right and left upper deltoid. CAM2038 weekly (Pharmaceutics International Inc) was provided in prefilled syringes with a subcutaneous injection volume of 0.48 mL (24 mg) and 0.64 mL (32 mg). An unblinded nurse (no other study involvement) injected CAM2038, rotating between right and left upper buttocks.
Pharmacodynamic Outcomes
The VAS scores were collected on pen and paper and assessed either on a 100-mm bipolar (ie, 50 = neutral response) or unipolar (ie, 0 = no effect) scale. At this time, drug liking and alertness/drowsiness VAS scores were bipolar, while any drug effects, good effects, high, bad effects, and desire to use opioids were unipolar. The VAS were administered at 30 minutes before drug administration and 5, 10, 15, 30, 45, 60, 75, 90, 120, 150, 180, 210, 240, 270, and 300 minutes after drug administration. The COWS and Objective Opioid Withdrawal Scales (OOWS) were administered before CAM2038 dosing and challenge sessions. Oral temperature, heart and respiratory rates, and blood pressure were collected before and 5 hours after dosing at regular intervals.
Safety outcomes included adverse event (AE) reports, physical examinations, vital signs, pulse oximetry, clinical laboratory assessments, and 12-lead electrocardiograms. Injection site examinations were conducted before and after CAM2038 injections. Pregnancy testing was conducted at screening, admission, and before each CAM2038 injection. Electrocardiograms were conducted before each CAM2038 injection. Depression was assessed during screening, before CAM2038, and on discharge day (Montgomery-Åsberg Depression Rating Scale).
Pharmacokinetic Sampling
Venous blood samples (6 mL) were collected on each CAM2038 injection day (before and 1, 4, 6, and 8 hours after injection), 1 hour before drug administration each test day (days 1-6 and days 8-13), and day 14 (approximately 168 hours after the second CAM2038 injection). Samples were centrifuged at 3000 rpm for 10 minutes at 20°C; plasma was stored (−20°C) until shipment to the laboratory (Worldwide Clinical Trials; Austin, Texas). Buprenorphine and norbuprenorphine were quantified with liquid chromatography mass spectrometry/mass spectrometry method validated for buprenorphine (range, 0.025-10 ng/mL) with a lower limit of quantification of 0.025 ng/mL and norbuprenorphine (range, 0.02-8 ng/mL) with a lower limit of quantification of 0.02 ng/mL.
Statistical Methods
Primary efficacy and safety analyses were conducted with the completer and randomized populations, respectively. The primary efficacy variable (drug liking VAS Emax), other VAS, and physiological measures were analyzed using a mixed model, including hydromorphone sequence, hydromorphone dose, period (first, second, or third day of each 3-day test session), test session (1-5), and dose by session interaction, with significance set at P < .05. Posthoc comparisons were made between the qualification phase and each posttreatment challenge for identical hydromorphone doses using Tukey t tests (eg, 18-mg hydromorphone qualification vs 18-mg hydromorphone session 1). Secondary analyses of these measures included Emax and minimum effect. In addition, estimated treatment effect, difference in treatment effect (hydromorphone test dose – placebo), and 95% CIs were determined to evaluate the a priori US Food and Drug Administration–defined blockade noninferiority criteria of 11 points. The OOWS and COWS were analyzed using a within-patient, repeated-measures 1-factor (study day 0 and thereafter) model. Safety data are summarized and reported descriptively. Analyses were conducted with SAS version 9.3 (SAS Institute).
Results
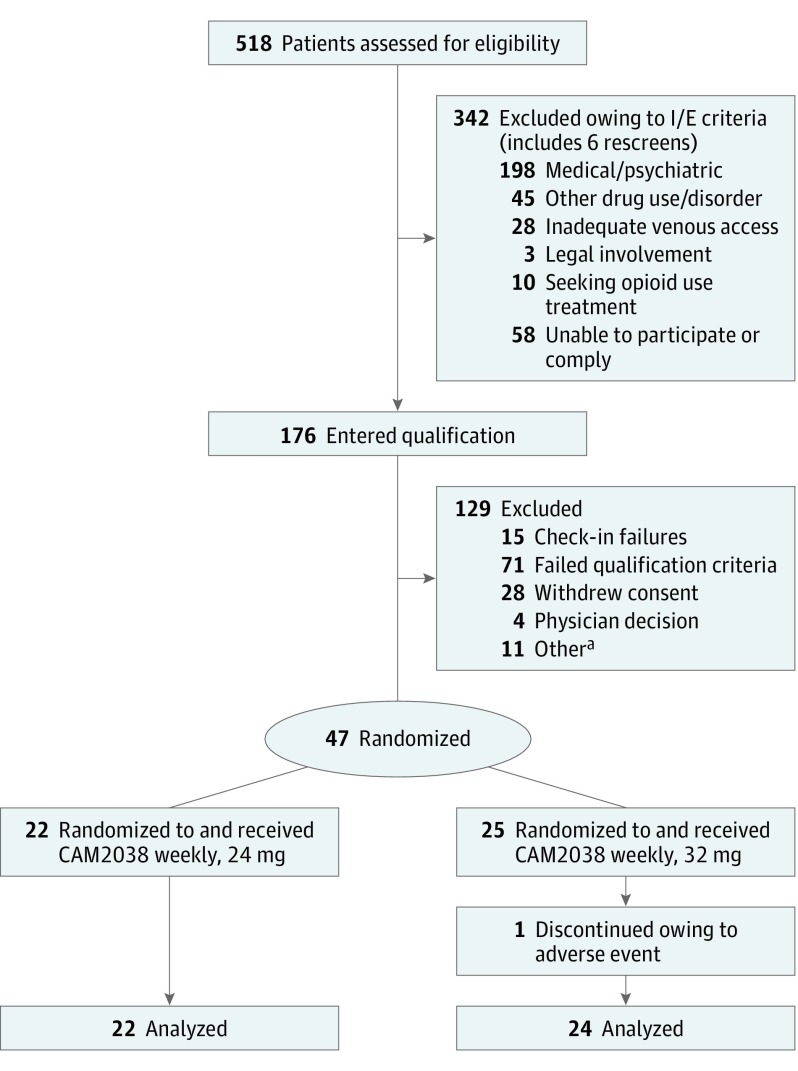
The CONSORT diagram summarizes participant disposition (Figure 1). Forty-seven participants (ie, safety population) were randomized and received CAM2038 weekly, 24 mg or 32 mg. One was discontinued owing to an AE (see Safety Outcomes section). Among the 46 completers, the mean [SD] age was 35.5 [9] years; 76% (n = 35) were male, the mean BMI was 24.6, and 50% were black, 47.8% were white, and 2.2% were other (reported by self-disclosure).
Figure 1. CONSORT Diagram Illustrates the Disposition of Patients From Screening Through Study Completion.
There had been 114 qualification phase failures. I/E indicates inclusion/exclusion.
aOther includes those leaving against medical advice or clinical laboratory and vital sign abnormalities.
Hydromorphone Challenges: Patient- and Observer-Rated Responses
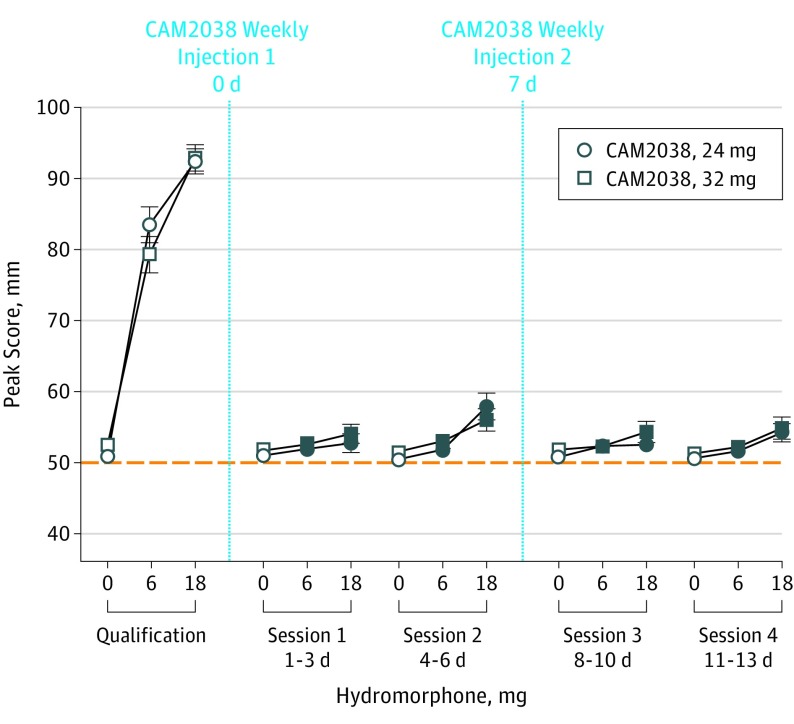
The primary outcome, drug liking Emax, is shown in Figure 2. During qualification, mean Emax scores for placebo were within the a priori–specified neutral range, while 6 and 18 mg produced dose-related increases in scores. Posthoc analyses revealed both active doses differed from placebo for both groups (P < .05). Treatment with CAM2038 weekly, 24 and 32 mg, suppressed response to hydromorphone as evidenced by (1) no active hydromorphone dose yielding a mean Emax score of 11 or greater compared with placebo (the a priori definition for complete blockade, including upper bounds of the 95% CI; Table), and (2) statistically significant differences between pretreatment vs posttreatment hydromorphone matched-dose comparisons (see Figure 2 legend for statistical outcomes). However, there were individual scores that exceeded the 11-point margin (Table). One patient was qualified in error but is included because a sensitivity analysis demonstrated no difference in outcomes.
Figure 2. Mean (±1 SEM) Maximum Effect Visual Analog Scale Scores for the Primary Outcome Measure of Drug Liking by Challenge Session for CAM2038 at 24 and 32 mg.
The dotted line denotes that this was a bipolar scale. Each hydromorphone dose response challenge (0, 6, and 18 mg; intramuscular) was conducted over a 3-day period, with the randomized dose order fixed within patient. Significant interaction effects for dose by session were found for drug liking (F8,308 = 74; P < .001 and F8,338 = 56.5; P < .001 for CAM2038, 24 mg and 32 mg, respectively). The filled symbols indicate a significant difference between that specific active hydromorphone dose (either 6 or 18 mg) during the qualification phase vs postrandomization challenges (Tukey t test; P < .05). CAM2038, 24 mg: n = 22; 32 mg: n = 24.
Table. Blocking Effects of 18-mg and 6-mg Hydromorphone Challenges With CAM2038 Weekly, 24 and 32 mg.
| Dosage | Time, d | Geometric Buprenorphine Concentration, Mean (SD), ng/mL | Drug Liking VAS Scores/Change From Placebo, Mean (95% CI) [Range], mm | |
|---|---|---|---|---|
| 18 mg vs 0 mg | 6 mg vs 0 mg | |||
| CAM2038, 24 mg | ||||
| Qualification session | −3 to −1 | 0a | 41.5 (37.8 to 45.2) [23 to 50] | 32.5 (27.2 to 37.9) [12 to 50] |
| Session 1 | 1 to 3 | 2.3 (0.92) | 1.8 (−1.1 to 4.6) [−4 to 29] | 1.0 (−0.3 to 2.2) [−3 to 8] |
| Session 2 | 4 to 6 | 1.49 (0.38) | 7.4 (3.4 to 11.4) [−2 to 28] | 1.4 (−0.1 to 2.8) [−2 to 12] |
| Session 3 | 8 to 10 | 2.66 (0.76) | 1.8 (−0.2 to 3.4) [−1 to 13] | 1.6 (−0.4 to 3.6) [−1 to 20] |
| Session 4 | 11 to 13 | 1.91 (0.37) | 3.6 (0.9 to 6.2) [−1 to 22] | 1.0 (−0.3 to 2.3) [−3 to 10] |
| CAM2038, 32 mg | ||||
| Qualification session | −3 to −1 | 0a | 40.4 (36.3 to 44.4) [21 to 49] | 26.8 (20.8 to 32.7) [−15 to 49] |
| Session 1 | 1 to 3 | 2.77 (1.37) | 2.4 (0.5 to 4.53) [−2 to 17] | 0.9 (−0.5 to 2.2) [−7 to 8] |
| Session 2 | 4 to 6 | 1.91 (0.63) | 4.5 (2.0 to 7.0) [−1 to 18] | 1.5 (−0.3 to 3.2) [−4 to 14] |
| Session 3 | 8 to 10 | 3.79 (1.56) | 2.5 (−0.3 to 5.3) [−4 to 29] | 0.5 (−0.2 to 1.2) [−4 to 4] |
| Session 4 | 11 to 13 | 2.44 (0.71) | 3.6 (0.5 to 6.7) [−1 to 29] | 0.9 (−0.1 to 1.9) [−3 to 9] |
Abbreviation: VAS, visual analog scale.
No samples were collected on days −3 to −1. This value is from baseline just prior to CAM2038 injection on day 0.
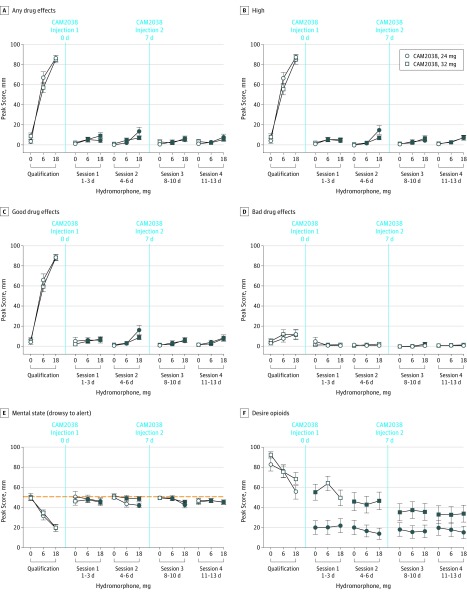
Figure 3 illustrates the secondary VAS measures. Significant dose by session interactions were found as follows: any effect (F8,308 = 66.3; P < .001 and F8,338 = 47.5; P < .001, for CAM2038, 24 mg and 32 mg, respectively), high (F = 64.6; P < .001 and F = 48.3; P < .001, for CAM2038, 24 mg and 32 mg, respectively), good effects (F = 51.2; P < .001 and F = 67.5; P < .001, for CAM2038, 24 mg and 32 mg, respectively), alertness/drowsiness (F = 4.1; P < .001 and F = 6.8; P < .001, for CAM2038, 24 mg and 32 mg, respectively), and significant main effect of dose for desire to use opioids (F2,308 = 3.7; P = .03 and F8,338 = 2.2; P = .03, for CAM2038, 24 mg and 32 mg, respectively). As with liking, hydromorphone produced robust dose-related increases in Emax scores during qualification for any effects, high, and good drug effects, and posthoc analyses revealed significant suppression of response to hydromorphone (with none exceeding the 11-point margin) after CAM2038. There were no significant effects of dose or dose by session for ratings of bad drug effects. For bipolar ratings of alertness/drowsiness, hydromorphone produced a clear dose-dependent increase in drowsiness during qualification that was effectively blocked by CAM2038. Ratings for desire to use opioids exhibited an atypical response pattern compared with the other VAS measures, with greater suppression by the lower CAM2038 dose (Figure 3).
Figure 3. Mean (±1 SEM) Maximum Effect for All but Alertness/Drowsiness (Minimum Effect) Visual Analog Scale Scores by Intramuscluar Hydromorphone Challenge Dose and Challenge Session for CAM2038 at 24 and 32 mg.
Significant dose by session interactions were found (see the Results section). The filled symbols indicate a significant difference between that specific active hydromorphone dose (either 6 or 18 mg) during the qualification phase vs postrandomization challenges (Tukey t test; P < .05 in all cases). The dotted line designates the bipolar scale. All other graphs are unipolar scales. CAM2038, 24 mg: n = 22; 32 mg: n = 24.
Opioid Withdrawal Suppression
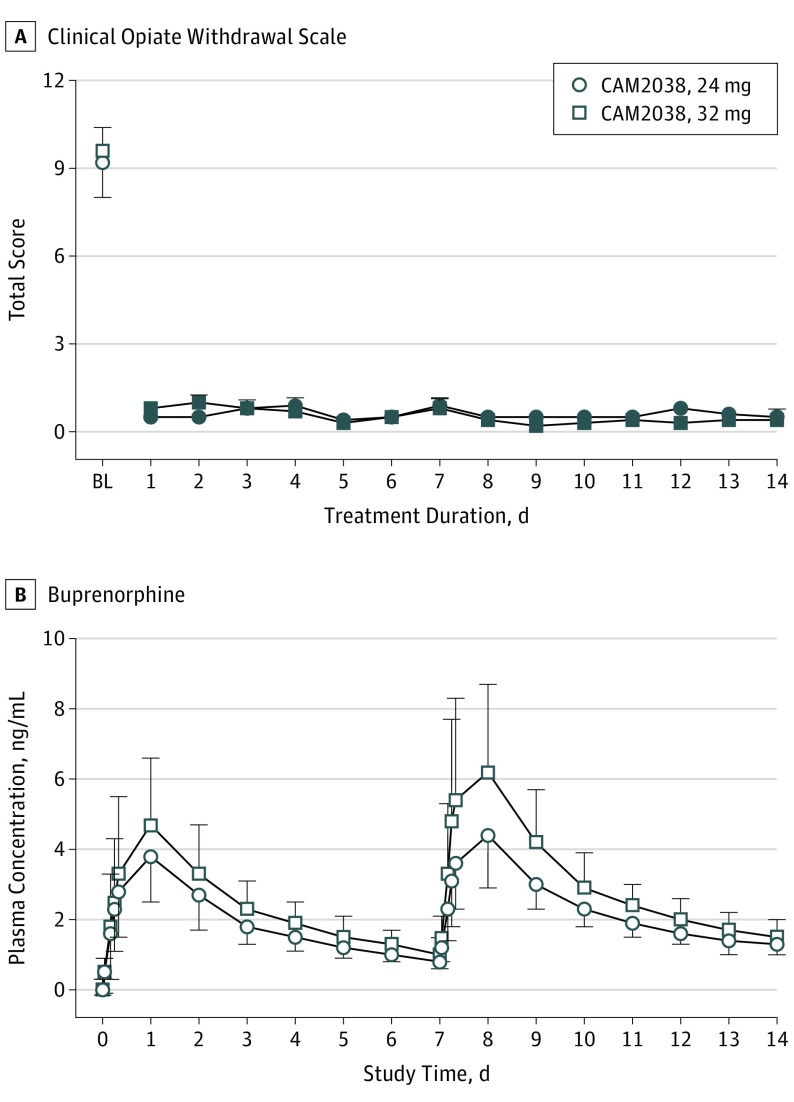
Figure 4A illustrates the COWS scores before and for 14 days after CAM2038 administration. Scores were modestly elevated at baseline during morphine stabilization, with a mean score of 9.4 before CAM2038. Owing to a protocol error at 1 site, the first 5 participants were treated before meeting COWS criteria (ie, ≥8). Excluding those, the mean preinjection COWS score was 11. Opioid withdrawal (ie, OOWS not shown) was completely suppressed on day 1 after CAM2038 and remained suppressed for the study duration (see the Figure 4 legend for statistical outcomes). Notably, there was no evidence of CAM2038 precipitating withdrawal in those 5 or any other participants.
Figure 4. Clinical Opiate Withdrawal Scale and Buprenorphine Concentrations .
A, Mean (±1 SEM) Peak Ratings for the Clinical Opiate Withdrawal Scale for CAM2038, 24 mg and 32 mg are shown at baseline (ie, before CAM2038 injection) and each study day thereafter. CAM2038 injections were administered on day 0 and day 7. The repeated-measures model revealed a significant main effect of day (all P < .001) for the Clinical Opiate Withdrawal Scale (F14,294 = 39 for 24 mg and F14,322 = 78.4 for 32 mg). B, Graph shows the arithmetic mean (±1 SD) buprenorphine plasma concentrations for the cohorts over the course of the study. CAM2038, 24 mg: n = 22; 32 mg: n = 24.
aThe filled symbols indicate a significant difference from the baseline score (Tukey t test; P < .05).
Physiological Outcomes
Hydromorphone produced significant and dose-dependent reductions in oxygen saturation during qualification (24 mg: F2,308 = 17.1, P < .001 and 32 mg: F2,338 = 12.3, P < .001) as follows: observed mean nadir of 96.5% (0 mg), 95.3% (6 mg), and 94.3% (18 mg). By comparison, the absolute reductions after CAM2038 treatment were as follows: 96.4% and 96.0% (0 mg), 95.6% and 96.0% (6 mg), and 95.3% and 95.5% (18 mg) for the CAM2038 24- and 32-mg doses, respectively. For respiratory rate, no main effects of hydromorphone dose were found and a dose by session interaction effect was identified for CAM2038, 24 mg, only (F8,308 = 2.5; P = .01). Maximal respiratory rate reductions were modest even during qualification (approximately 1 breath/minute).
No effects were observed on heart rate or systolic blood pressure. For peak minimum diastolic blood pressure in the CAM2038, 24 mg, group only, there was a significant dose session interaction effect (F8,308 = 3.6; P < .001), driven largely by a reliable reduction in resting pressure (collected after intramuscular placebo) after CAM2038 administration in comparison with higher values during qualification.
CAM2038 Pharmacokinetics
CAM2038 weekly produced dose-dependent buprenorphine (Figure 4B) and norbuprenorphine plasma concentration–time profiles (additional pharmacokinetic data in eTable 1 and eTable 2 in Supplement 2). Similar profiles were observed for the 2 groups with a median time-to-maximum plasma concentration of about 24 hours and an apparent terminal half-life of 4 to 5 days. The average buprenorphine concentration was 1.81 ng/mL and 2.29 ng/mL for the first and second 24-mg doses, respectively, and 2.24 ng/mL and 3.05 ng/mL for the corresponding first and second 32-mg doses, respectively. A 50% accumulation in trough concentration was observed for both groups between the first and second doses.
Safety Outcomes
During the study, 38 participants (81%) experienced 1 or more AEs (64% with 24 mg and 96% with 32 mg). Constipation (19%), injection-site pain (11%) and erythema (9%), headache (9%), and nausea (9%) were most common. Adverse events suspected to be related to CAM2038 were reported by 57.4%, including constipation (19%), injection-site pain and erythema (9% each), and headache (6%), with most rated as mild severity. One case of ventricular extrasystoles in a patient receiving CAM2038, 32 mg, resulted in discontinuation. One patient assigned to CAM2038, 32 mg, exhibited abnormal liver function test results at discharge and was subsequently diagnosed as having hepatitis C; neither was considered related to CAM2038 (eTable 3 in Supplement 2).
Discussion
This study examined the efficacy of weekly CAM2038 at doses of 24 and 32 mg, corresponding to approximately 16 and 24 mg of SL buprenorphine, to produce opioid blockade and suppress opioid withdrawal. Both doses produced immediate and sustained suppression of responses to hydromorphone for the weekly interval between CAM2038 injections and met the a priori US Food and Drug Administration–designated criteria for complete opioid blockade. Withdrawal was also completely suppressed after the first injection and for the study duration. The pharmacokinetic data reveal that CAM2038 produced dose-dependent plasma concentrations of buprenorphine with accumulation across successive injections, and another recent study suggests that steady state may be achieved after 4 CAM2038 weekly injections.
Modeling studies have suggested that a plasma concentration from 2 to 3 ng/mL (translating to ≥70% mu-opioid receptor occupancy) is needed to produce significant opioid blockade. The Table shows that, with only 1 exception, mean plasma concentrations fell within this range after CAM2038, 32 mg. However, of the individuals who had scores exceeding the 11-point margin, 25% of those instances were accompanied by concentrations of 2 ng/mL or more. Thus, plasma concentrations may be useful predictors for blockade but are not absolute. The variability in scores suggests that a few individuals may experience partial blockade, especially early in treatment. The hydromorphone doses (6 and 18 mg) used here are estimated to be equivalent to parenteral morphine at approximately 40 and 121 mg, respectively; mean responses showed complete blockade after initial CAM2038 injection of both doses and throughout the dosing interval. The overall greatest response observed (drug liking Emax difference between 18 mg vs 0 mg with CAM2038, 24 mg) was during session 2 with an upper bound of the 95% CI at 9.6 and a lower observed mean plasma concentration of approximately 1.25 ng/mL. This concentration was reached within 4 hours after CAM2038 injection, supporting the observed rapid onset of opioid blockade. A similar pattern of rapid-onset, robust, and sustained blockade was observed for other VAS measures of abuse potential (Figure 4).
During treatment initiation, it is important that withdrawal symptoms are well-controlled. The COWS and OOWS scores were reduced to near zero on the first dosing day with suppression thereafter. Baseline values were comparatively low because participants were morphine maintained and abstinent for fewer than 15 hours. Buprenorphine alone can precipitate withdrawal in individuals physically dependent on opioids owing to its partial agonist profile; therefore, in clinical practice, it is important to avoid initiating buprenorphine under specified conditions (eg, dosing too close to last opioid use and introducing buprenorphine at high doses). Guidelines recommend abstaining from opioids for a period sufficient for modest withdrawal to emerge before administration of an initial low dose (eg, 4 mg) and followed by another small dose if the first was well-tolerated. Here, participants were stabilized with morphine, a short-acting opioid with a half-life similar to commonly abused opioids (eg, heroin and oxycodone), doses were omitted for fewer than 15 hours, and modest opioid withdrawal was typically present, closely mimicking clinical practice procedures. Importantly, CAM2038 weekly treatment did not require induction and stabilization with SL buprenorphine. With no cases of precipitated withdrawal observed with this approach, it seems likely that patients could be inducted directly with CAM2038 using standard clinical procedures.
Limitations
One study limitation was the absence of a placebo-controlled group; however, it would not be possible to have an untreated opioid-dependent group retained without treatment and would be unsafe to test these high opioid doses in a nontolerant population. Owing to a protocol error that may have been somewhat serendipitous, some participants received their first CAM2038 injection with little to no evidence of withdrawal (COWS score <8); none experienced precipitated withdrawal. The pharmacokinetic profile of CAM2038, with its gradual increasing buprenorphine plasma concentrations reaching maximum concentration at approximately 24 hours, may be a de facto induction procedure, mimicking recommended SL buprenorphine induction procedures starting with a low dose repeatedly administered over the first day. Both weekly and monthly CAM2038 has been shown to produce dose-proportional plasma concentrations of buprenorphine in the clinically relevant range of current SL buprenorphine treatments but without the daily peaks and troughs of these products, potentially providing more stable protection against withdrawal emergence.
Conclusions
Weekly and monthly buprenorphine subcutaneous depots are being developed for individualized treatment of patients with OUD to address the limitations of currently marketed once-daily SL formulations, including misuse, diversion, and nonadherence. CAM2038 produced clinically relevant buprenorphine plasma levels, translating into rapid and sustained opioid blockade and withdrawal suppression, and was well-tolerated both systemically and locally. Findings suggest that CAM2038 formulations will be effective in reducing illicit opioid use and relapse, while eliminating the risk for misuse and diversion.
Trial Protocol
eTable 1. Summary of Plasma Buprenorphine Pharmacokinetic Parameters for CAM2038 q1w 24 mg and CAM2038 q1w 32 mg (Completer Population).
eTable 2. Summary of Plasma Norbuprenorphine Pharmacokinetic Parameters for CAM2038 q1w 24 mg and CAM2038 q1w 32 mg (Completer Population).
eTable 3. Treatment-Emergent Adverse Events Occurring in at Least 5% of Patients in Amy CAM2038 q1w Treatment Group and Overall by MedDRA System Organ Class and MedDRA Preferred Term (Safety Population).
References
- 1.United Nations Office of Drugs and Crime (UNODC) World Drug Report: United Nations Publication, Sale No. E.16.XI.7. New York, NY: United Nations; 2016. [Google Scholar]
- 2.United Nations Office of Drugs and Crime (UNODC) World Drug Report: United Nations Publication, Sale No. E.14.XI.7. New York, NY: United Nations; 2014. [Google Scholar]
- 3.Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267(20):2750-2755. [PubMed] [Google Scholar]
- 4.Ling W, Charuvastra C, Collins JF, et al. . Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction. 1998;93(4):475-486. [DOI] [PubMed] [Google Scholar]
- 5.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54(8):713-720. [DOI] [PubMed] [Google Scholar]
- 6.Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Comparison of buprenorphine and methadone in the treatment of opioid dependence. Am J Psychiatry. 1994;151(7):1025-1030. [DOI] [PubMed] [Google Scholar]
- 7.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-Year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361(9358):662-668. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274(1):361-372. [PubMed] [Google Scholar]
- 9.Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther. 1988;247(1):47-53. [PubMed] [Google Scholar]
- 10.Comer SD, Walker EA, Collins ED. Buprenorphine/naloxone reduces the reinforcing and subjective effects of heroin in heroin-dependent volunteers. Psychopharmacology (Berl). 2005;181(4):664-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35(4):501-516. [DOI] [PubMed] [Google Scholar]
- 12.Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207(4431):657-659. [DOI] [PubMed] [Google Scholar]
- 13.Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winstock AR, Lea T, Sheridan J. Prevalence of diversion and injection of methadone and buprenorphine among clients receiving opioid treatment at community pharmacies in New South Wales, Australia. Int J Drug Policy. 2008;19(6):450-458. [DOI] [PubMed] [Google Scholar]
- 15.Alho H, Sinclair D, Vuori E, Holopainen A. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78. [DOI] [PubMed] [Google Scholar]
- 16.Comer SD, Collins ED. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther. 2002;303(2):695-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middleton LS, Nuzzo PA, Lofwall MR, Moody DE, Walsh SL. The pharmacodynamic and pharmacokinetic profile of intranasal crushed buprenorphine and buprenorphine/naloxone tablets in opioid abusers. Addiction. 2011;106(8):1460-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comer SD, Sullivan MA, Vosburg SK, et al. . Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin abusers. Addiction. 2010;105(4):709-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovegrove MC, Mathew J, Hampp C, Governale L, Wysowski DK, Budnitz DS. Emergency hospitalizations for unsupervised prescription medication ingestions by young children. Pediatrics. 2014;134(4):e1009-e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes BD, Klein-Schwartz W, Doyon S. Toxicity of buprenorphine overdoses in children. Pediatrics. 2008;121(4):e782-e786. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Buprenorphine prescribing practices and exposures reported to a poison center: Utah, 2002-2011. MMWR Morb Mortal Wkly Rep. 2012;61(49):997-1001. [PubMed] [Google Scholar]
- 22.Tiberg F, Johnsson M, Nistor C, Joabsson F. Self-assembling liquid formulations In: Wright JC, Burgess DJ, eds. Long-Acting Injections and Implants. New York, NY: Springer; 2012:315-334. [Google Scholar]
- 23.Tiberg F, Johnsson M, Jankunec M, Barauskas J. Phase behavior, functions, and medical applications of soy phosphatidylcholine and diglyceride lipid compositions. Chem Lett. 2012;41(10):1090-1092. doi: 10.1246/cl.2012.1090 [DOI] [Google Scholar]
- 24.Albayaty M, Linden M, Olsson H, Johnsson M, Strandgården K, Tiberg F. Pharmacokinetic evaluation of once-weekly and once-monthly buprenorphine subcutaneous injection depots (CAM2038) vs intravenous and sublingual buprenorphine in healthy volunteers under naltrexone blockade: an open label phase 1 study. Adv Ther. 2017;34(2):560-575. [DOI] [PubMed] [Google Scholar]
- 25.Midlothian Laboratories Buprenorphine hydrochloride-buprenorphine hydrochloride tablet: final package insert. 2010.
- 26.Ciraulo DA, Hitzemann RJ, Somoza E, et al. . Pharmacokinetics and pharmacodynamics of multiple sublingual buprenorphine tablets in dose-escalation trials. J Clin Pharmacol. 2006;46(2):179-192. [DOI] [PubMed] [Google Scholar]
- 27.Greenwald MK, Johanson CE, Moody DE, et al. . Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28(11):2000-2009. [DOI] [PubMed] [Google Scholar]
- 28.Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: the utility and limitations of research instruments In: First MB, ed. Standardized Evaluation in Clinical Practice. Washington, DC: American Psychiatric Publishing Inc; 2003:103-130. [Google Scholar]
- 30.Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35(2):253-259. [DOI] [PubMed] [Google Scholar]
- 31.Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13(3):293-308. [DOI] [PubMed] [Google Scholar]
- 32.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Bonson KR. An equivalence test for the comparison between a test drug and placebo in human abuse potential studies. J Biopharm Stat. 2013;23(2):294-306. [DOI] [PubMed] [Google Scholar]
- 34.Nasser AF, Heidbreder C, Gomeni R, Fudala PJ, Zheng B, Greenwald MK. A population pharmacokinetic and pharmacodynamic modelling approach to support the clinical development of RBP-6000, a new, subcutaneously injectable, long-acting, sustained-release formulation of buprenorphine, for the treatment of opioid dependence. Clin Pharmacokinet. 2014;53(9):813-824. [DOI] [PubMed] [Google Scholar]
- 35.Gutstein HB, Akil H. Opioid analgesics In: Brunton LL, Laso JS, Parker KL, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th ed New York, NY: McGraw-Hill; 2006:547-590. [Google Scholar]
- 36.Walsh SL, June HL, Schuh KJ, Preston KL, Bigelow GE, Stitzer ML. Effects of buprenorphine and methadone in methadone-maintained subjects. Psychopharmacology (Berl). 1995;119(3):268-276. [DOI] [PubMed] [Google Scholar]
- 37.Stoller KB, Bigelow GE, Walsh SL, Strain EC. Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology (Berl). 2001;154(3):230-242. [DOI] [PubMed] [Google Scholar]
- 38.Whitley SD, Sohler NL, Kunins HV, et al. . Factors associated with complicated buprenorphine inductions. J Subst Abuse Treat. 2010;39(1):51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Center for Substance Abuse Treatment (CSAT) Clinical Guidelines for Buprenorphine in the Treatment of Opioid Addiction: Vol (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Summary of Plasma Buprenorphine Pharmacokinetic Parameters for CAM2038 q1w 24 mg and CAM2038 q1w 32 mg (Completer Population).
eTable 2. Summary of Plasma Norbuprenorphine Pharmacokinetic Parameters for CAM2038 q1w 24 mg and CAM2038 q1w 32 mg (Completer Population).
eTable 3. Treatment-Emergent Adverse Events Occurring in at Least 5% of Patients in Amy CAM2038 q1w Treatment Group and Overall by MedDRA System Organ Class and MedDRA Preferred Term (Safety Population).