This multicenter study examines the association between the occurrence of small nodal tumor infiltrates and bone marrow micrometastases as well as their prognostic relevance.
Key Points
Question
What association exists between disseminated tumor cells in lymph nodes and bone marrow of patients with stage I to III colon cancer, and what is its prognostic significance?
Findings
This prospective multicenter study of 122 patients with stage I to III colon cancer demonstrates that disseminated tumor cells in lymph nodes and bone marrow are not associated with each other. Furthermore, disseminated tumor cells in the lymph nodes and bone marrow are associated with worse prognosis.
Meaning
Lymphogenic and hematogenic tumor cell dissemination are of equal importance already at early disease stages, questioning the traditional idea of sequential metastatic spread.
Abstract
Importance
Small nodal tumor infiltrates (SNTI; isolated tumor cells and micrometastases) in sentinel lymph nodes and bone marrow micrometastases (BMM) were independently described as prognostic factors in patients with colon cancer.
Objective
To examine the association between the occurrence of SNTI and BMM as well as their prognostic relevance.
Design, Setting, and Participants
This prospective study was conducted at 3 university-affiliated institutions in Switzerland between May 2000 and December 2006. Statistical analyses were performed in October 2016. A total of 122 patients with stage I to III colon cancer were included. Follow-up time exceeded 6 years, with no patients lost to follow-up.
Interventions
Bone marrow aspiration from the iliac crests and in vivo sentinel lymph node mapping were performed during open standard oncological resection. Bone marrow aspirates were stained with the pancytokeratin marker A45-B/B3. All sentinel lymph nodes underwent multilevel sectioning and were stained with hematoxylin-eosin and the pancytokeratin marker AE1/AE3.
Main Outcomes and Measures
Association of SNTI in sentinel lymph nodes and BMM in patients with stage I to III colon cancer and the prognostic effect on disease-free survival (DFS) and overall survival (OS).
Results
Of the 122 patients, 63 (51.6%) were female, with a mean (SD) age of 71.2 (11.7) years. Small nodal tumor infiltrates and BMM were found in a total of 21 patients (17.2%) and 46 patients (37.7%), respectively. The occurrence of BMM was not associated with the presence of SNTI by standard correlation (κ, −0.07; 95% CI, −0.29 to 0.14; P = .49) nor by univariate logistic regression analysis (odds ratio, 0.64; 95% CI, 0.22-1.67; P = .37) or multivariate logistic regression analysis (odds ratio, 1.09; 95% CI, 0.34-3.28; P = .88). The presence of SNTI was an independent negative prognostic factor for DFS (hazard ratio [HR], 2.93; 95% CI, 1.24-6.93; P = .02) and OS (HR, 4.04; 95% CI, 1.56-10.45; P = .005), as was BMM (HR, 2.07; 95% CI, 1.06-4.06; P = .04; and HR, 2.68; 95% CI, 1.26-5.70; P = .01; respectively). The combined detection of BMM and SNTI demonstrated the poorest DFS (HR, 6.73; 95% CI, 2.29-19.76; P = .006) and OS (HR, 5.96; 95% CI, 1.66-21.49; P = .03).
Conclusions and Relevance
This study demonstrates no association between the occurrence of SNTI and BMM in patients with stage I to III colon cancer. However, both SNTI and BMM are independent negative prognostic factors regarding DFS and OS, and the occurrence of both is associated with significantly worse prognosis compared with either one of them.
Trial Registration
clinicaltrials.gov Identifier: NCT00826579
Introduction
Standard treatment of colon cancer involves surgical resection according to oncological principles and adjuvant chemotherapy in stage III and high-risk stage I and II disease. The goal of adjuvant chemotherapy is to eradicate systemic micrometastatic disease not visible in the diagnostic workup procedures at the time of operation. This concept leads to a roughly 20% recurrence reduction in patients with node-positive (ie, stage III) colon cancer. For most patients with node-negative disease (ie, stage I and II), surgery is regarded to be the curative treatment. However, despite this curative attempt, up to 30% of patients with node-negative disease will develop disease recurrence, most likely owing to missed micrometastatic disease at initial tumor staging. Pathological standard processing with hematoxylin-eosin entails a considerable risk of missing micrometastases (ie, tumor deposits with a diameter of 0.2 mm to ≤2 mm, labeled as pN1[mi]) and isolated tumor cells (ITC; single tumor cells or clusters of tumor cells of 0.2 mm or less in diameter; pN0[i+]) in lymph nodes.
In previous reports, we have demonstrated that implementation of sentinel lymph node (SLN) mapping with intensive histopathological workup results in the detection of these small nodal tumor infiltrates (SNTI) in up to one-quarter of patients with node-negative colon cancer. Mounting evidence indicates that SNTI are associated with an increased risk of disease recurrence and death in so-called node-negative patients. Similarly, small tumor infiltrates are found in the bone marrow of up to one-third of patients with stage I to III colon cancer and are also associated with decreased survival, as most recently demonstrated by our group. However, to our knowledge, the association between disseminated tumor cells in lymph nodes and the bone marrow has not been investigated to date. The objective of the present study was 2-fold: first, to examine the association of SNTI in SLN with bone marrow micrometastases (BMM) in patients with stage I to III colon cancer; and second, to assess their prognostic effect on disease-free survival (DFS) and overall survival (OS).
Methods
Study Settings
This prospective study (clinicaltrials.gov identifier: NCT00826579) was performed at 3 university-affiliated hospitals in Switzerland (University Hospital Basel, Basel; Hospital Center Biel/Bienne, Biel/Bienne; and Cantonal Hospital Olten, Olten) from May 2000 until December 2006. The study protocol was approved by the ethical committees of all participating centers and was conducted in accordance with the Declaration of Helsinki. Inclusion and exclusion criteria of the study population have been reported previously. In brief, patients with preoperatively verified stage I to III colon cancer met the inclusion criteria. Exclusion criteria for SLN mapping and bone marrow aspiration were stage IV disease, rectal cancer, history of other solid malignancies, prior abdominal cancer surgery, documented allergy to isosulfan blue, and current pregnancy or breast feeding. Written informed consent was obtained from all patients prior to surgery.
In all patients, open colon cancer resection according to oncological principles was performed. Tumors were staged according to the Union for International Cancer Control (UICC) TNM Classification of Malignant Tumors, 6th edition. The SLN mapping and bone marrow aspiration have been described in detail elsewhere. Briefly, bone marrow was aspirated from both iliac crests after induction of general anesthesia and before the start of the operation. Bone marrow aspirates were processed as described by our group previously. Detection of 1 or more tumor cells was considered as bone marrow–positive. For the SLN mapping, isosulfan blue was injected in vivo into the subseroa around the tumor. Lymph nodes staining blue within 10 minutes after dye application were marked with a suture and collected as SLN and then processed according to our previously reported protocol. The protocol consists of 5 serial sections of each SLN that were obtained at 3 different representative levels. The first section of each level was stained with hematoxylin-eosin. If no metastatic deposits were detectable by hematoxylin-eosin, the fourth section of each level was immunostained with the pancytokeratin marker AE1/AE3 (DakoCytomation). If micrometastases were detected by hematoxylin-eosin or immunohistochemistry, node-negative patients were upstaged to stage III (pN1[mi]). If ITC were detected in the SLN, patients were not upstaged according to the TNM Classification of Malignant Tumors, 6th edition (pN0[i+]).
All pathologists were blinded regarding the tumor histology. Adjuvant chemotherapy was recommended for patients with stage III cancer and to patients with stage II cancer featuring high-risk factors (ie, less than 12 lymph nodes analyzed, T4 tumor, lymphovascular or perineural invasion, poorly differentiated histology, or tumor perforation). Bone marrow positivity was not considered an indication for adjuvant chemotherapy. Follow-up was performed according to the national surveillance guidelines for patients after curative colon cancer resection.
Statistical Analyses
Statistical analyses were performed in October 2016 with the R statistical software version 3.2.5 (The R Foundation). A 2-sided P value < .05 was considered statistically significant. χ2 statistics were used to analyze proportions and analysis of variance tests to analyze continuous variables. The association between SNTI and BMM was assessed by κ statistics with 95% CIs. Additionally, univariable and multivariable logistic regression analyses were performed to analyze the predictive value of SNTI for BMM and of BMM for SNTI. Because of complete and quasicomplete separation (occurrence empty categories), Firths correction to the likelihood (penalized maximum likelihood) was applied. In logistic regression, P values were computed by likelihood-ratio tests, and Wald-type CIs were estimated. The effect of SNTI and BMM as prognostic factors for disease-specific survival and OS was assessed in univariable and multivariable Cox regression analyses. For Cox regression, P values were computed by likelihood-ratio tests, and Wald-type CIs were estimated. The entire analysis was repeated in the cohort of patients with node-negative stage I and II cancer as a sensitivity analysis.
Results
In a total of 122 patients, in vivo SLN mapping and bone marrow aspiration were successfully performed. No adverse events occurred, neither with in vivo SLN mapping nor with bone marrow aspiration. Overall, SNTI and BMM were detected in 21 patients (17.2%) and 46 patients (37.7%), respectively. Of the patients with SNTI in the SLN, 2 showed micrometastases and 19 showed ITC. According to the presence of SNTI and BMM, 4 subgroups of patients were identified; there were 61 SNTI-negative (SNTI−), BMM-negative (BMM−) patients; 15 SNTI-positive (SNTI+), BMM− patients; 40 SNTI−, BMM-positive (BMM+) patients, and 6 SNTI+, BMM+ patients. Patient and tumor characteristics are listed in Table 1. A mean (interquartile range) of 24.0 (18.2-32.0) lymph nodes and 3.0 (2.0-5.0) SLN were collected per patient over a median follow-up of 74.2 months.
Table 1. Baseline Data for the 4 Groups of Patients With Stage I to III Colon Cancer.
| Variable | Total (n = 122) | SNTI−, BMM− (n = 61) | SNTI+, BMM− (n = 15) | SNTI−, BMM+ (n = 40) | SNTI+, BMM+ (n = 6) | P Value |
|---|---|---|---|---|---|---|
| Sex, No. (%) | .21a | |||||
| Male | 59 (48.4) | 26 (42.6) | 11 (73.3) | 19 (47.5) | 3 (50.0) | |
| Female | 63 (51.6) | 35 (57.4) | 4 (26.7) | 21 (52.5) | 3 (50.0) | |
| Age, y | .86b | |||||
| Median (IQR) | 73.6 (65.8-78.9) | 73.6 (65.8-78.1) | 73.6 (66.1-77.5) | 73.5 (64.7-82.0) | 73.7 (71.3-76.4) | |
| Mean (SD) | 71.2 (11.7) | 70.4 (12.1) | 71.7 (9.2) | 71.8 (12.9) | 73.9 (3.2) | |
| Range | 27.3-92.4 | 27.3-88.2 | 55.5-84.2 | 38.3-92.4 | 70.2-78.1 | |
| Validated, No. (%) | 122 (100) | 61 (100) | 15 (100) | 40 (100) | 6 (100) | |
| BMI | .38b | |||||
| Median (IQR) | 25.8 (22.9-28.4) | 25.8 (22.9-28.2) | 24.3 (22.1-27.6) | 26.3 (23.5-29.7) | 25.4 (25.3-27.0) | |
| Mean (SD) | 26.1 (4.5) | 25.8 (4.1) | 25.0 (4.4) | 27.1 (5.3) | 25.8 (2.0) | |
| Range | 15.9-44.3 | 18.3-34.5 | 18.5-33.0 | 15.9-44.3 | 22.9-28.6 | |
| Validated, No. (%) | 120 (98.4) | 60 (98.4) | 15 (100) | 39 (97.5) | 6 (100) | |
| Tumor localization, No. (%) | .01a | |||||
| Right | 44 (36.1) | 18 (29.5) | 2 (13.3) | 23 (57.5) | 1 (16.7) | |
| Transversum | 27 (22.1) | 13 (21.3) | 6 (40.0) | 7 (17.5) | 1 (16.7) | |
| Left | 51 (41.8) | 30 (49.2) | 7 (46.7) | 10 (25.0) | 4 (66.7) | |
| pT stage, No. (%) | .95a | |||||
| T1 | 8 (6.6) | 4 (6.6) | 1 (6.7 | 3 (7.5) | 0 | |
| T2 | 18 (14.8) | 10 (16.4) | 1 (6.7) | 6 (15.0) | 1 (16.7) | |
| T3 | 81 (66.4) | 41 (67.2) | 10 (66.7) | 25 (62.5) | 5 (83.3) | |
| T4 | 15 (12.3) | 6 (9.8) | 3 (20.0) | 6 (15.0) | 0 | |
| pN stage, No. (%) | .45a | |||||
| N0 | 78 (63.9) | 40 (65.6) | 9 (60.0) | 27 (67.5) | 2 (33.3) | |
| N1 | 30 (24.6) | 13 (21.3) | 4 (26.7) | 11 (27.5) | 2 (33.3) | |
| N2 | 14 (11.5) | 8 (13.1) | 2 (13.3) | 2 (5.0) | 2 (33.3) | |
| Grade, No. (%) | .11a | |||||
| G2 | 86 (70.5) | 46 (75.4) | 13 (86.7) | 23 (57.5) | 4 (66.7) | |
| G3 | 36 (29.5) | 15 (24.6) | 2 (13.3) | 17 (42.5) | 2 (33.3) | |
| Lymphvasc invasion, No. (%) | .08a | |||||
| No | 98 (80.3) | 52 (85.2) | 10 (66.7) | 33 (82.5) | 3 (50.0) | |
| Yes | 24 (19.7) | 9 (14.8) | 5 (33.3) | 7 (17.5) | 3 (50.0) | |
| CEA, preoperation | .78b | |||||
| Median (IQR) | 2.0 (1.1-5.4) | 2.3 (1.1-5.4) | 1.6 (1.2-4.8) | 1.8 (1.0-5.6) | 1.9 (1.3-4.1) | |
| Mean (SD) | 4.5 (7.0) | 4.9 (7.9) | 5.6 (9.8) | 3.8 (4.3) | 3.6 (3.9) | |
| Range | 0-41.0 | 0-41.0 | 0.6-38.0 | 0-15.0 | 0.8-11.0 | |
| Validated, No. (%) | 112 (91.8) | 53 (86.9) | 15 (100) | 38 (95.0) | 6 (100) | |
| CEA, postoperation | .87b | |||||
| Median (IQR) | 1.1 (0.7-1.8) | 1.1 (0.7-1.8) | 1.0 (0.7-1.2) | 1.2 (0.8-2.0) | 0.7 (0.6-1.7) | |
| Mean (SD) | 1.6 (1.8) | 1.8 (2.5) | 1.2 (0.9) | 1.6 (1.1) | 1.3 (1.2) | |
| Range | 0.5-12.9 | 0.5-12.9 | 0.5-3.2 | 0.5-4.8 | 0.5-2.7 | |
| Validated, No. (%) | 58 (47.5) | 24 (39.3) | 8 (53.3) | 23 (57.5) | 3 (50.0) | |
| Chemotherapy, No. (%) | .45a | |||||
| No | 82 (67.2) | 44 (72.1) | 8 (53.3) | 27 (67.5) | 3 (50.0) | |
| Yes | 40 (32.8) | 17 (27.9) | 7 (46.7) | 13 (32.5) | 3 (50.0) | |
| BM positive, No. (%) | <.001a | |||||
| No | 76 (62.3) | 61 (100) | 15 (100) | 0 | 0 | |
| Yes | 46 (37.7) | 0 | 0 | 40 (100) | 6 (100) | |
| No. of positive cells in BM | .003b | |||||
| Median (IQR) | 0 (0-1.0) | NA | NA | 2.0 (1.0-4.5) | 4.0 (3.2-4.0) | |
| Mean (SD) | 2.4 (9.5) | NA | NA | 6.7 (15.7) | 4.2 (2.0) | |
| Range | 0-95.0 | NA | NA | 1.0-95.0 | 2.0-8.0 | |
| Validated, No. (%) | 122 (100) | 61 (100) | 15 (100) | 40 (100) | 6 (100) | |
| SLN No. | .41a | |||||
| Median (IQR) | 3.0 (2.0-4.8) | 3.0 (2.0-5.0) | 4.0 (2.5-4.5) | 3.0 (1.0-4.0) | 2.5 (2.0-4.5) | |
| Mean (SD) | 3.3 (2.1) | 3.5 (2.2) | 3.9 (2.4) | 2.9 (1.8) | 3.0 (1.7) | |
| Range | 1.0-10.0 | 1.0-9.0 | 1.0-10.0 | 1.0-8.0 | 1.0-5.0 | |
| Validated, No. (%) | 122 (100) | 61 (100) | 15 (100) | 40 (100) | 6 (100) | |
| Positive SLN (by hematoxylin-eosin) | .47a | |||||
| Median (IQR) | 0 (0-0) | 0 (0-0) | 0 (0-0.5) | 0 (0-0) | 0 (0-0.8) | |
| Mean (SD) | 0.3 (0.8) | 0.3 (0.8) | 0.6 (1.4) | 0.2 (0.6) | 0.5 (0.8) | |
| Range | 0-5.0 | 0-4.0 | 0-5.0 | 0-3.0 | 0-2.0 | |
| Validated, No. (%) | 122 (100) | 61 (100) | 15 (100) | 40 (100) | 6 (100) | |
| Positive SLN (by IHC) | <.001a | |||||
| Median (IQR) | 0 (0-0) | 0 (0-0) | 1.0 (1.0-1.0) | 0 (0-0) | 1.0 (1.0-1.0) | |
| Mean(SD) | 0.2 (0.6) | 0 (0.1) | 1.2 (0.9) | 0 (0) | 1.3 (0.8) | |
| Range | 0-4.0 | 0-1.0 | 0-4.0 | 0 | 1.0-3.0 | |
| Validated, No. (%) | 122 (100) | 61 (100) | 15 (100) | 40 (100) | 6 (100) | |
| No. LN | .38a | |||||
| Median (IQR) | 24.0 (18.2-32.0) | 24.0 (18.0-33.0) | 22.0 (18.5-25.5) | 25.5 (21.5-36.0) | 18.5 (16.5-19.8) | |
| Mean (SD) | 26.7 (11.7) | 26.6 (10.8) | 26.0 (14.2) | 28.4 (12.6) | 19.5 (6.7) | |
| Range | 7.0-62.0 | 7.0-56.0 | 14.0-62.0 | 7.0-62.0 | 12.0-32.0 | |
| Validated, No. (%) | 122 (100) | 61 (100) | 15 (100) | 40 (100) | 6 (100) | |
| SNTI, No. (%) | <.001b | |||||
| No SNTI | 101 (82.8) | 61 (100) | NA | 40 (100) | 0 | |
| SNTI | 21 (17.2) | 0 | 15 (100) | 0 | 6 (100) |
Abbreviations: BM, bone marrow; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BMM, bone marrow micrometastases; CEA, carcinoembryonic antigen; IHC, immunohistochemistry; IQR, interquartile range; LN, lymph node; NA, not applicable; pN, pathological lymph node stage; pT, pathological tumor stage; SLN, sentinel lymph node; SNTI, small nodal tumor infiltrates; +, positive; −, negative.
By χ2 test, Monte Carlo simulated.
By analysis of variance test.
Association of SNTI and BMM
An association between the presence of SNTI and BMM was not observed in either the entire cohort (κ, −0.07; 95% CI, −0.29 to 0.14; P = .49) or the subset of patients with stage I and II cancer (κ, −0.13; 95% CI, −0.4 to 0.14; P = .34). The occurrence of SNTI did not predict the occurrence of BMM in univariable logistic regression (odds ratio [OR], 0.64; 95% CI, 0.22-1.67; P = .37) or in multivariable logistic regression (OR, 1.09; 95% CI, 0.34-3.28; P = .88) (Table 2). Similarly, the occurrence of BMM did not predict the occurrence of SNTI in univariable logistic regression (OR, 0.64; 95% CI, 0.22-1.67; P = .37) or multivariable logistic regression (OR, 1.19; 95% CI, 0.38-3.64; P = .77) (Table 2). When limiting the analysis to patients with node-negative stage I and II cancer, similar results were obtained for the prediction of BMM by SNTI (univariate analysis: OR, 0.39; 95% CI, 0.07-1.50; P = .18; multivariate analysis: OR, 0.58; 95% CI, 0.09-2.95; P = .52) and for the prediction of SNTI by BMM (univariate analysis: OR, 0.39; 95% CI, 0.07-1.50; P = .18; multivariate analysis: OR, 0.74; 95% CI, 0.11-3.86; P = .73). Right-sided tumor location was predictive for BMM compared with transverse location (univariate analysis: HR, 0.36; 95% CI, 0.13-0.97; P = .02; multivariate analysis: HR, 0.22; 95% CI, 0.07-0.67; P = .005) or left-sided location (univariate analysis: HR, 0.32; 95% CI, 0.14-0.74; P = .02; multivariate analysis: HR, 0.27; 95% CI, 0.10-0.66; P = .005) (Table 2). Further predictors of SNTI were a higher UICC stage as well as lymphovascular invasion in univariate (UICC stage: stage II: hazard ratio [HR], 11.37; 95% CI, 1.37-1483.96; stage III: HR, 13.7; 95% CI, 1.62-1793.63; P = .03; lymphovascular invasion: HR, 3.26; 95% CI, 1.16-1.57; P = .03) and multivariate analysis (UICC stage: stage II: HR, 12.83; 95% CI, 1.5-1682.45; stage III: HR, 8.57; 95% CI, 0.92-1141.67; P = .051; lymphovascular invasion: HR, 3.58; 95% CI, 1.01-14.14; P = .048) (Table 2). When considering only node-negative patients, right-sided tumor location was predictive for BMM compared with transverse location (univariate analysis: HR, 0.20; 95% CI, 0.03-0.83; P = .02; multivariate analysis: HR, 0.20; 95% CI, 0.03-0.87; P = .03) or left-sided location (univariate analysis: HR, 0.31; 95% CI, 0.11-0.83; P = .02; multivariate analysis: HR, 0.29; 95% CI, 0.09-0.85; P = .02). Higher UICC stage (univariate analysis: HR, 11.37; 95% CI, 1.37-1483.95; P = .02; multivariate analysis: HR, 10.27; 95% CI, 1.10-1727.27; P = .04) and lymphovascular invasion (univariate analysis: HR, 8.47; 95% CI, 1.84-40.32; P = .007; multivariate analysis: HR, 10.44; 95% CI, 1.24-151.56; P = .03) remained predictive of SNTI in node-negative patients.
Table 2. Prediction of Bone Marrow Micrometastases and Small Nodal Tumor Infiltrates in Logistic Regression.
| Variable | Univariate, OR (95% CI)a | P Value | Multivariate, OR (95% CI)a | P Value |
|---|---|---|---|---|
| Prediction of BMM | ||||
| SNTI | .37 | .88 | ||
| No SNTI | [Reference] | [Reference] | ||
| SNTI in SLN | 0.64 (0.22-1.67) | 1.09 (0.34-3.28) | ||
| Tumor localization | .02 | .005 | ||
| Right colon | [Reference] | [Reference] | ||
| Transverse | 0.36 (0.13-0.97) | 0.22 (0.07-0.67) | ||
| Left colon | 0.32 (0.14-0.74) | 0.27 (0.10-0.66) | ||
| UICC/AJCC stage | .90 | .51 | ||
| I | [Reference] | [Reference] | ||
| II | 0.80 (0.30-2.19) | 0.63 (0.20-1.89) | ||
| III | 0.90 (0.33-2.56) | 1.04 (0.33-3.36) | ||
| Grade | .03 | .04 | ||
| G2 | [Reference] | [Reference] | ||
| G3 | 2.41 (1.10-5.53) | 2.68 (1.06-7.11) | ||
| Lymphovascular invasion | .64 | .91 | ||
| No | [Reference] | [Reference] | ||
| Yes | 1.24 (0.50-3.02) | 1.07 (0.33-3.32) | ||
| Prediction of SNTI | ||||
| BMM | .37 | .77 | ||
| No BMM | [Reference] | [Reference] | ||
| BMM | 0.64 (0.22-1.67) | 1.19 (0.38-3.64) | ||
| Tumor localization | .06 | .14 | ||
| Right colon | [Reference] | [Reference] | ||
| Transverse | 4.34 (1.16-19.48) | 4.76 (0.97-27.39) | ||
| Left colon | 0.37 (1.02-14.01) | 2.78 (0.75-12.51) | ||
| UICC/AJCC stage | .03 | .05 | ||
| I | [Reference] | [Reference] | ||
| II | 11.37 (1.37-1483.96) | 12.83 (1.5-1682.43) | ||
| III | 13.70 (1.62-1793.63) | 8.57 (0.92-1141.67) | ||
| Grade | .28 | .03 | ||
| G2 | [Reference] | [Reference] | ||
| G3 | 0.55 (0.16-1.57) | 0.24 (0.05-0.90) | ||
| Lymphovascular invasion | .03 | .05 | ||
| No | [Reference] | [Reference] | ||
| Yes | 3.26 (1.16-8.91) | 3.58 (1.01-14.14) | ||
Abbreviations: AJCC, American Joint Committee on Cancer; BMM, bone marrow micrometastases; OR, odds ratio; SLN, sentinel lymph node; SNTI, small nodal tumor infiltrates; UICC, Union for International Cancer Control.
Odds ratio with 95% CIs from univariate and multivariate Firths logistic regression fitted penalized maximum likelihood with P value from likelihood ratio tests.
Survival Analysis
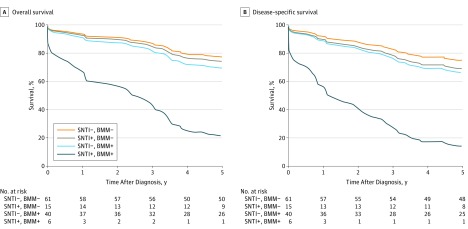
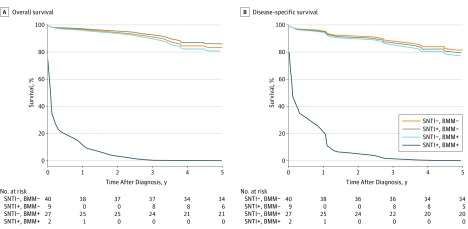
The presence of SNTI (SNTI+, BMM−) or BMM (SNTI−, BMM+) and especially the combined occurrence (SNTI+, BMM+) was associated with adverse DFS (HR, 1.28; 95% CI, 0.52-3.18; HR, 1.42; 95% CI, 0.74-2.72; and HR, 6.73; 95% CI, 2.29-19.76; P = .006) and OS (HR, 1.16; 95% CI, 0.43-3.14; HR, 1.42; 95% CI, 0.73-2.77; and HR, 5.96; 95% CI, 1.66-21.49; P = .03) (Figure 1). These results were also confirmed when only patients with stage I or II cancer were considered (Figure 2). Five-year DFS rates were 78.7% (95% CI, 69.1-89.7) for SNTI−, BMM− patients, 58.7% (95% CI, 38.0-90.6) for SNTI+, BMM− patients, 65.0 (95% CI, 51.8-81.6) for SNTI−, BMM+ patients, and 16.7% (95% CI, 2.8-99.7) for SNTI+, BMM+ patients; 5-year OS rates were 82.0% (95% CI, 72.9-92.2), 66.0% (95% CI, 45.7-95.4), 67.4% (95% CI, 54.3-83.7), and 16.7% (95% CI, 2.8-99.7), respectively. When only patients with stage I and II cancer were considered, 5-year DFS rates for node-negative patients were 85.0% (95% CI, 74.6-96.8) for SNTI−, BMM− patients, 63.5% (95% CI, 37.7-100%) for SNTI+, BMM− patients, 74.1% (95% CI, 59.3-92.6) for SNTI−, BMM+ patients, and 0% for SNTI+, BMM+ patients; 5-year OS rates were 85.0% (95% CI, 74.6-96.8), 76.2% (95% CI, 52.1-100), 77.8% (95% CI, 63.6-95.2), and 0%, respectively.
Figure 1. Kaplan-Meier Curve for Overall and Disease-Specific Survival in Patients With Stage I to III Colon Cancer.
According to the presence of small nodal tumor infiltrates (SNTI) and bone marrow micrometastases (BMM), there were 4 subgroups of patients, including 61 SNTI-negative (SNTI−), BMM-negative (BMM−) patients (reference group); 15 SNTI-positive (SNTI+), BMM− patients (overall survival [OS]: hazard ratio [HR], 1.16; 95% CI, 0.43-3.14; disease-specific survival [DSS]: HR, 1.28; 95% CI, 0.52-3.18); 40 SNTI−, BMM-positive (BMM+) patients (OS: HR, 1.42; 95% CI, 0.73-2.77; DSS: HR, 1.42; 95% CI, 0.74-2.72), and 6 SNTI+, BMM+ patients (OS: HR, 5.96; 95% CI, 1.66-21.49; DSS: HR, 6.73; 95% CI, 2.29-19.76) (likelihood ratio test: OS, P = .03; DSS, P = .006).
Figure 2. Kaplan-Meier Curve for Overall and Disease-Specific Survival in Patients With Stage I to II Colon Cancer.
According to the presence of small nodal tumor infiltrates (SNTI) and bone marrow micrometastases (BMM), there were 4 subgroups of patients, including 40 SNTI-negative (SNTI−), BMM-negative (BMM−) patients (reference group); 9 SNTI-positive (SNTI+), BMM− patients (overall survival [OS]: hazard ratio [HR], 0.82; 95% CI, 0.19-3.61; disease-specific survival [DSS]: HR, 1.12; 95% CI, 0.33-3.82); 27 SNTI−, BMM-positive (BMM+) patients (OS: HR, 1.16; 95% CI, 0.47-2.86; DSS: HR, 1.25; 95% CI, 0.53-2.96), and 2 SNTI+, BMM+ patients (OS: HR, 61.60; 95% CI, 17.69-214.52; DSS: HR, 34.55; 95% CI, 10.30-115.85) (likelihood ratio test: OS, P = .03; DSS, P = .052).
Small nodal tumor infiltrates were a negative prognostic factor for DFS in univariate analysis (HR, 2.01; 95% CI, 1.04-3.87; P = .05) and multivariate analysis (HR, 2.93; 95% CI, 1.24-6.93; P = .02) and for OS in multivariate regression analysis (HR, 4.04; 95% CI, 1.56-10.45; P = .005) (Table 3). In multivariate analysis, BMM were identified as a negative prognostic factor for DFS (HR, 2.07; 95% CI, 1.06-4.06; P = .04) and OS (HR, 2.68; 95% CI, 1.26-5.70; P = .01) (Table 3). When considering the subgroup of stage I and II patients, SNTI and BMM were no longer independent prognostic factors (data not shown).
Table 3. Cox Regression for Overall Survival.
| Variable | Univariable Analysis, HR (95% CI) | P Value | Multivariable Analysis, HR (95% CI) | P Value |
|---|---|---|---|---|
| BMM | .15 | .01 | ||
| No | [Reference] | [Reference] | ||
| Yes | 1.57 (0.85-2.89) | 2.68 (1.26-5.70) | ||
| SNTI | .11 | .005 | ||
| No | [Reference] | [Reference] | ||
| Yes | 1.87 (0.92-3.80) | 4.04 (1.56-10.45) | ||
| Tumor localization | .14 | .05 | ||
| Right | [Reference] | [Reference] | ||
| Transverse | 2.29 (1.01-5.19) | 1.04 (0.37-2.86) | ||
| Left | 1.60 (0.76-3.37) | 2.81 (1.06-7.42) | ||
| Stage | .16 | .001 | ||
| I | [Reference] | [Reference] | ||
| II | 0.65 (0.28-1.50) | 0.29 (0.09-0.90) | ||
| III | 1.26 (0.57-2.79) | 1.71 (0.56-5.21) | ||
| Grade | .02 | .12 | ||
| G2 | [Reference] | [Reference] | ||
| G3 | 2.14 (1.15-3.97) | 2.18 (0.82-5.81) | ||
| Lymphovascular invasion | .002 | .14 | ||
| No | [Reference] | [Reference] | ||
| Yes | 3.08 (1.61-5.92) | 2.14 (0.78-5.85) | ||
| CEA, preoperation, mg | .15 | .37 | ||
| 0-3 | [Reference] | [Reference] | ||
| ≥3 | 1.13 (0.59-2.16) | 1.43 (0.63-3.25) | ||
| Not perf | 0.23 (0.03-1.70) | 0.32 (0.04-2.89) | ||
| LN analyzed | .39 | .02 | ||
| ≥12 | [Reference] | [Reference] | ||
| 7-11 | 2.78 (0.38-20.32) | 58.90 (3.72-933.33) | ||
| Sex | .42 | .41 | ||
| Male | [Reference] | [Reference] | ||
| Female | 0.78 (0.42-1.43) | 1.39 (0.64-3.03) | ||
| Age, y | <.001 | .02 | ||
| <70 | [Reference] | [Reference] | ||
| ≥70 | 4.47 (1.88-10.62) | 2.99 (1.10-8.11) | ||
| BMI | .07 | .05 | ||
| <25 | [Reference] | [Reference] | ||
| ≥25 | 0.56 (0.31-1.04) | 0.51 (0.25-1.01) | ||
| Adjuvant chemotherapy | .006 | <.001 | ||
| No | [Reference] | [Reference] | ||
| Yes | 0.36 (0.16-0.81) | 0.06 (0.02-0.23) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BMM, bone marrow micrometastases; CEA, carcinoembryonic antigen; HR, hazard ratio; LN, lymph nodes; SNTI, small nodal tumor infiltrates.
Discussion
The present investigation provides compelling evidence that tumor cell dissemination to the SLN and to the bone marrow are independent events in patients with colon cancer. Moreover, while both SNTI and BMM are independent negative prognostic factors regarding DFS and OS, the combined occurrence is associated with significantly worse prognosis compared with either one of them.
In our cohort, SNTI were detected in almost one-fifth of patients considered to be lymph node–negative with conventional histopathological examination. Bone marrow micrometastases were detected in more than one-third of patients. These detection rates compare favorably with previously published results by our and other groups. Interestingly, only a very small subgroup of patients showed simultaneous occurrence of SNTI and BMM. Similar observations were made in patients with early breast cancer and melanoma, reporting simultaneous lymphatic and hematogenic dissemination in one-fifth of patients. Two of these studies assessed the correlation of micrometastases in the SLN and the bone marrow in patients with early breast cancer and could not detect any association. According to the common concepts of cancer growth and progression, earliest tumor cell dissemination is thought to initially follow the lymphatic vessels toward the first draining lymph nodes prior to systemic (ie, hematogenic) spread. The observations made in the present study directly challenge this concept in colon cancer. Although hematogenic tumor cell spread has been reported in early stages of colon cancer, its connection with lymphatic dissemination and the chronology of these events have never been fully understood. The presented results demonstrate that tumor cell dissemination is not restricted to one typical sequence and therefore call into question the very concept of sequential tumor cell spread.
In the present cohort, patients with SNTI or BMM had a significantly decreased DFS and OS that was even worse when SNTI and BMM occurred simultaneously in the same patient (Figure 1 and Figure 2). Both SNTI and BMM were identified as independent negative prognostic markers. However, as the detection of SNTI has no therapeutic consequence in patients with stage III colon cancer, we performed a separate analysis with node-negative patients (ie, with stage I and II cancer) only. It revealed that SNTI and/or BMM were still associated with decreased survival but were no longer independent prognostic markers below the level of statistical significance, most likely owing to the smaller sample size. Mounting evidence suggests that SNTI are indeed a negative prognostic marker in stage I and II colon cancer. By contrast, the literature regarding the prognostic significance of BMM is very scarce but suggests an association with negative survival. In addition, a 2015 study detected a negative prognostic effect of circulating tumor cells obtained from peripheral blood samples in patients with stage I to III colorectal cancer. This study compares favorably with the present results, as tumor cell dissemination to the peripheral blood and the bone marrow both represent hematogenous spread.
The growing evidence raises the question whether patients with disseminated tumor cells might benefit from adjuvant chemotherapy. The current TNM Classification of Malignant Tumors, 7th edition, recommends adjuvant chemotherapy for patients with lymph node micrometastases but not for patients with ITC or BMM. With the change from the 5th to the 6th edition of the TNM Classification of Malignant Tumors in 2002, ITC were newly allocated as pN0 and micrometastases as pN1. Remarkably, the discrimination between micrometastases and ITC is chosen randomly at 0.2 mm and not made on the basis of measured survival differences. Because of such ambiguities, pathological and oncological practice differs significantly even between countries in Western Europe. Germany and Switzerland have adopted the TNM Classification of Malignant Tumors, 7th edition, classifying ITC as pN0 and offering adjuvant chemotherapy to patients with micrometastases staged as pN1. Conversely, in the United Kingdom, colon cancer is staged according to the TNM Classification of Malignant Tumors, 5th edition. Any metastatic deposit in the lymph node is staged as pN1, suggesting adjuvant chemotherapy for patients with ITC and micrometastases. In the Netherlands, colon cancer is staged according to the TNM Classification of Malignant Tumors, 5th edition, but ITC and micrometastases are staged as pN0, not suggesting adjuvant chemotherapy for these patients. These essential differences in colon cancer treatment are highly unsatisfactory and emerged mainly because of an inconclusively held debate. It is therefore of utmost importance to keep this debate going and to further seek clarification.
Limitations
Our study had limitations. First, this is a cohort study and not a randomized clinical trial, and some confounders may therefore be present. However, baseline characteristics between the patient groups are comparable, and the presence of a relevant selection bias is unlikely. Second, the lower patient number for the stage I and II subset analysis was most probably responsible for the lack of significant results for the association of SNTI as well as BMM with survival in this subgroup. However, the negative prognostic effect of SNTI and BMM was highly significant for stage I to III cancer. Finally, the determination of mismatch repair protein expression and microsatellite instability were not standard procedures at the time of patient inclusion, and we therefore cannot report these data. Regardless, to our knowledge, this study is the first in the literature to investigate the association between SNTI and BMM in patients with colon cancer.
Conclusions
Our results clearly demonstrate that hematogenic and lymphogenic tumor cell dissemination are already of equal importance at early disease stages. Therefore, the traditional idea of sequential metastatic spread must be seriously doubted. These findings also raise the question if standard lymph node dissection and evaluation are sufficiently accurate to identify patients at risk of disease recurrence. Our results suggest that lymph node dissection may only play a subordinate role and that more meticulous techniques, like SLN mapping, bone marrow aspiration and/or semiautomatic messenger RNA amplification, will be of higher importance for the detection of tumors’ real metastatic potential and for individual patient treatment.
References
- 1.André T, de Gramont A, Vernerey D, et al. . Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol. 2015;33(35):4176-4187. [DOI] [PubMed] [Google Scholar]
- 2.André T, Boni C, Mounedji-Boudiaf L, et al. ; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators . Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343-2351. [DOI] [PubMed] [Google Scholar]
- 3.Rahbari NN, Bork U, Motschall E, et al. . Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30(1):60-70. [DOI] [PubMed] [Google Scholar]
- 4.Sirop S, Kanaan M, Korant A, et al. . Detection and prognostic impact of micrometastasis in colorectal cancer. J Surg Oncol. 2011;103(6):534-537. [DOI] [PubMed] [Google Scholar]
- 5.Hermanek P, Hutter RV, Sobin LH, Wittekind C. International Union Against Cancer: classification of isolated tumor cells and micrometastasis. Cancer. 1999;86(12):2668-2673. [PubMed] [Google Scholar]
- 6.Viehl CT, Guller U, Cecini R, et al. . Sentinel lymph node procedure leads to upstaging of patients with resectable colon cancer: results of the Swiss prospective, multicenter study sentinel lymph node procedure in colon cancer. Ann Surg Oncol. 2012;19(6):1959-1965. [DOI] [PubMed] [Google Scholar]
- 7.Bembenek A, Schneider U, Gretschel S, Fischer J, Schlag PM. Detection of lymph node micrometastases and isolated tumor cells in sentinel and nonsentinel lymph nodes of colon cancer patients. World J Surg. 2005;29(9):1172-1175. [DOI] [PubMed] [Google Scholar]
- 8.Viehl CT, Guller U, Hamel CT, et al. . Carbon dye staining of sentinel lymph nodes facilitates microstaging of colon cancer patients. World J Surg. 2006;30(3):453-456. [DOI] [PubMed] [Google Scholar]
- 9.Weixler B, Warschkow R, Güller U, et al. . Isolated tumor cells in stage I & II colon cancer patients are associated with significantly worse disease-free and overall survival. BMC Cancer. 2016;16:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weixler B, Warschkow R, Zettl A, et al. . Intranodal mapping using carbon dye results in more accurate lymph node staging in colon cancer patients. World J Surg. 2015;39(10):2583-2589. [DOI] [PubMed] [Google Scholar]
- 11.Mescoli C, Albertoni L, Pucciarelli S, et al. . Isolated tumor cells in regional lymph nodes as relapse predictors in stage I and II colorectal cancer. J Clin Oncol. 2012;30(9):965-971. [DOI] [PubMed] [Google Scholar]
- 12.Viehl CT, Weixler B, Guller U, et al. . Presence of bone marrow micro-metastases in stage I-III colon cancer patients is associated with worse disease-free and overall survival. Cancer Med. 2017;6(5):918-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobin LH, Wittekind C, eds. TNM Classification of Malignant Tumors. 6th ed Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- 14.Viehl CT, Hamel CT, Marti WR, et al. . Identification of sentinel lymph nodes in colon cancer depends on the amount of dye injected relative to tumor size. World J Surg. 2003;27(12):1285-1290. [DOI] [PubMed] [Google Scholar]
- 15.Langer I, Guller U, Worni M, et al. ; Swiss Multicenter Sentinel Lymph Node Study Group in Breast Cancer . Bone marrow micrometastases do not impact disease-free and overall survival in early stage sentinel lymph node-negative breast cancer patients. Ann Surg Oncol. 2014;21(2):401-407. [DOI] [PubMed] [Google Scholar]
- 16.Criblez D. Follow-up care after resection of colorectal polypes and colorectal cancer [in German]. Schweiz Arzteztg. 2001;2001(82):1967-1971. [Google Scholar]
- 17.Watson PF, Petrie A. Method agreement analysis: a review of correct methodology. Theriogenology. 2010;73(9):1167-1179. [DOI] [PubMed] [Google Scholar]
- 18.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27-38. doi: 10.2307/2336755 [DOI] [Google Scholar]
- 19.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21(16):2409-2419. [DOI] [PubMed] [Google Scholar]
- 20.van der Pas MH, Meijer S, Hoekstra OS, et al. . Sentinel-lymph-node procedure in colon and rectal cancer: a systematic review and meta-analysis. Lancet Oncol. 2011;12(6):540-550. [DOI] [PubMed] [Google Scholar]
- 21.Saha S, Bilchik A, Wiese D, et al. . Ultrastaging of colorectal cancer by sentinel lymph node mapping technique: a multicenter trial. Ann Surg Oncol. 2001;8(9, suppl):94S-98S. [PubMed] [Google Scholar]
- 22.Leong SP, Tseng WW. Micrometastatic cancer cells in lymph nodes, bone marrow, and blood: clinical significance and biologic implications. CA Cancer J Clin. 2014;64(3):195-206. [DOI] [PubMed] [Google Scholar]
- 23.Saha S, Ali S, Ghanem M, et al. . Comparative analysis of bone marrow micrometastases with sentinel lymph node status in early-stage breast cancer. Ann Surg Oncol. 2009;16(2):276-280. [DOI] [PubMed] [Google Scholar]
- 24.Trocciola SM, Hoda S, Osborne MP, et al. . Do bone marrow micrometastases correlate with sentinel lymph node metastases in breast cancer patients? J Am Coll Surg. 2005;200(5):720-725. [DOI] [PubMed] [Google Scholar]
- 25.Hellmann S. Karnosfky Memorial Lecture: natural history of small breast cancers. J Clin Oncol. 2012;12:2229-2234. [DOI] [PubMed] [Google Scholar]
- 26.Weitz J, Kienle P, Magener A, et al. . Detection of disseminated colorectal cancer cells in lymph nodes, blood and bone marrow. Clin Cancer Res. 1999;5(7):1830-1836. [PubMed] [Google Scholar]
- 27.Bork U, Rahbari NN, Schölch S, et al. . Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br J Cancer. 2015;112(8):1306-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faerden AE, Sjo OH, Bukholm IRK, et al. . Lymph node micrometastases and isolated tumor cells influence survival in stage I and II colon cancer. Dis Colon Rectum. 2011;54(2):200-206. [DOI] [PubMed] [Google Scholar]
- 29.Leinung S, Würl P, Weiss CL, Röder I, Schönfelder M. Cytokeratin-positive cells in bone marrow in comparison with other prognostic factors in colon carcinoma. Langenbecks Arch Surg. 2000;385(5):337-343. [DOI] [PubMed] [Google Scholar]
- 30.Sobin LH, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumors. 7th ed Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 31.Sobin LH, Fleming ID; Union Internationale Contre le Cancer and the American Joint Committee on Cancer . TNM Classification of Malignant Tumors, fifth edition (1997). Cancer. 1997;80(9):1803-1804. [DOI] [PubMed] [Google Scholar]
- 32.Quirke P, Williams GT, Ectors N, Ensari A, Piard F, Nagtegaal I. The future of the TNM staging system in colorectal cancer: time for a debate? Lancet Oncol. 2007;8(7):651-657. [DOI] [PubMed] [Google Scholar]
- 33.Ong MLH, Schofield JB. Assessment of lymph node involvement in colorectal cancer. World J Gastrointest Surg. 2016;8(3):179-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantel K, Schlimok G, Braun S, et al. . Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J Natl Cancer Inst. 1993;85(17):1419-1424. [DOI] [PubMed] [Google Scholar]
- 35.Juhl H, Stritzel M, Wroblewski A, et al. . Immunocytological detection of micrometastatic cells: comparative evaluation of findings in the peritoneal cavity and the bone marrow of gastric, colorectal and pancreatic cancer patients. Int J Cancer. 1994;57(3):330-335. [DOI] [PubMed] [Google Scholar]
- 36.Sobin LH, Wittekind C, eds. TNM: Classification of Malignant Tumours. 5th ed Hoboken, NJ: John Wiley & Sons; 1997. [Google Scholar]
- 37.Comprehensive Cancer Centre the Netherlands What to find on Oncoline. http://www.oncoline.nl/colorectaalcarcinoom. Accessed October 15, 2016.
- 38.Güller U, Zettl A, Worni M, et al. . Molecular investigation of lymph nodes in colon cancer patients using one-step nucleic acid amplification (OSNA): a new road to better staging? Cancer. 2012;118(24):6039-6045. [DOI] [PMC free article] [PubMed] [Google Scholar]