Abstract
Severe alpha-1 antitrypsin (AAT) deficiency is one of the most common serious genetic diseases in adults of European descent. Individuals with AAT deficiency have a greatly increased risk for emphysema and liver disease. Other manifestations include bronchiectasis, necrotizing panniculitis and granulomatosis with polyangiitis. Despite the frequency and potential severity, AAT deficiency remains under-recognized, and there is often a delay in diagnosis. This review will focus on three recent updates that should serve to encourage testing and diagnosis of AAT deficiency: first, the publication of a randomized clinical trial demonstrating the efficacy of intravenous augmentation therapy in slowing the progression of emphysema in AAT deficiency; second, the mounting evidence showing an increased risk of lung disease in heterozygous PI MZ genotype carriers; last, the recent publication of a clinical practice guideline, outlining diagnosis and management. Though it has been recognized for more than fifty years, AAT deficiency exemplifies the modern paradigm of precision medicine, with a diagnostic test that identifies a genetic subtype of a heterogeneous disease, leading to a targeted treatment.
Keywords: alpha-1 antitrypsin deficiency, diagnosis, testing, COPD
Introduction
Severe alpha-1 antitrypsin (AAT) deficiency is one of the most common serious genetic diseases in adults of European descent, with an estimated prevalence of 1 in 1500 to 1 in 3000 1, 2. The AAT protein, the major inhibitor of neutrophil elastase, is encoded by the SERPINA1 gene on chromosome 14 and secreted from the liver. Severe AAT deficiency is usually due to autosomal recessive inheritance of the PI*Z allele of SERPINA1, with the resulting PI ZZ genotype. Severe AAT deficiency is associated with an increased risk of emphysema, especially in cigarette smokers, due to the imbalance between proteases and anti-proteases in the lung tissue. The misfolded Z protein polymerizes in the liver, causing damage to hepatocytes and potentially leading to cirrhosis and hepatocellular carcinoma. Other conditions associated with AAT deficiency include bronchiectasis, necrotizing panniculitis and granulomatosis with polyangiitis.
Despite the high prevalence and potentially serious clinical manifestations, AAT deficiency remains under-recognized, and there is often a delay in diagnosis in symptomatic individuals, as long as five to eight years 3, 4. This review will focus on three recent updates that should serve to encourage testing and diagnosis of AAT deficiency, which is necessary to appropriately counsel patients and provide specific therapy. First is the publication of a randomized clinical trial demonstrating the efficacy of intravenous augmentation therapy in slowing the progression of emphysema in patients with chronic obstructive pulmonary disease (COPD) due to AAT deficiency 5. Second is the mounting evidence showing an increased risk of airflow obstruction in heterozygous carriers (genotype PI MZ) 6, 7. Last is the recent publication of a clinical practice guideline outlining the diagnosis and management of AAT deficiency 8. The full spectrum of the genetics, biology, and clinical manifestations of AAT deficiency has been reviewed elsewhere 9– 12.
Clinical trial of augmentation therapy
Augmentation therapy with intravenous infusion of pooled human AAT is available in North America and in several, but not all, European countries. Augmentation therapy was approved based on studies demonstrating biochemical efficacy 13, 14. Clinical use has been supported largely from observational and registry studies 15– 17. Two small randomized trials suggested a reduction in the loss of lung tissue on chest computed tomography scans 18, 19, which was statistically significant when the results were combined in a meta-analysis 20.
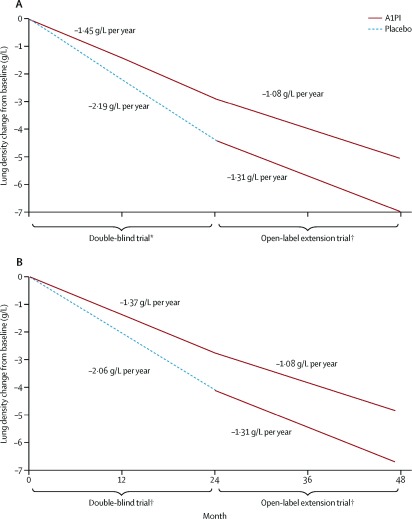
These studies provided the background for the RAPID trial, a multicenter, double-blind, randomized controlled trial of intravenous AAT augmentation therapy 5. Study subjects were former smokers ages 18–65 with severe AAT deficiency (<11 µM) and reduced lung function with a forced expiratory volume in 1 second (FEV 1) of 35–70% predicted. 95 subjects received AAT infusions at a dose of 60 mg/kg weekly, and 87 subjects received placebo infusions. The primary endpoint was lung density on chest CT scans at total lung capacity (TLC) and functional residual capacity combined, over the two year trial. There was a 29% reduction in the change of combined lung density endpoint between the two arms, which was not statistically significant. However, the change in lung density at TLC was 34% lower in the AAT augmentation group ( P = 0.03) ( Figure 1). Lung density measurements at full inspiration (i.e. TLC) are the clinical standard, may be subject to less noise 21, and more closely reflect emphysema 22, 23. Emphysema progression as an endpoint is more specific to the disease pathology of AAT deficiency than FEV 1 decline, a more commonly used outcome in COPD clinical trials. Augmentation therapy could be considered as disease-modifying, and was estimated to prolong time to death or lung transplant by almost six years, based on post-hoc extrapolation 5, 24. There was no difference in change in lung function, exacerbations, exercise capacity, or quality of life between the treatment and placebo arms.
Figure 1. The RAPID trial of augmentation therapy in severe alpha-1 antitrypsin deficiency.
Intravenous augmentation therapy slowed the loss of lung density on chest CT scans over four years: ( A) all subjects and ( B) subjects completing the open-label extension study. Reprinted from 5, with permission from Elsevier.
Following the two-year RAPID randomized trial, subjects from both arms were offered augmentation therapy in a two-year open label extension trial 24. Both the group which received AAT augmentation therapy in the randomized trial (early-start) and the group which received placebo (delayed-start) showed the same rate of decline in lung density. However, subjects initially treated with placebo did not recover any of the lost lung density. The extension study showed that the effects of augmentation were sustained over four years and supported earlier treatment initiation.
Patients in RAPID with higher trough serum AAT levels during treatment had less loss of lung density in a post-hoc analysis. A major reason for the increased levels was a greater infused dose of augmentation in patients with higher body weight 5. This finding has motivated clinical trials of double dose weekly augmentation therapy 25. Other therapies under investigation include inhaled AAT 26 and gene therapy 27. Based on the RAPID trial, augmentation therapy currently can be recommended in this targeted subgroup of patients with severe AAT deficiency and reduced lung function in a potentially modifiable range (e.g. FEV 1 35–70% predicted).
COPD risk in heterozygous carriers
Starting shortly after the discovery that severe AAT deficiency was associated with COPD 28, there has been a debate regarding the risk of lung disease in PI MZ carriers. Most of the literature consisted of small case-control studies, many of which found an association. We performed a meta-analysis, finding an increased risk of COPD with a combined odds ratio (OR) of 2.31 (95% CI 1.60-3.35). The effect was attenuated in studies that adjusted for cigarette smoking (OR 1.61) 29. However, population-based studies showed no difference in FEV 1 between normal genotype (PI MM) and PI MZ individuals. These results were consistent with a small increase in COPD risk in all PI MZ heterozygotes or a larger risk in a subset.
Several pivotal studies have been published subsequently. Sørheim and colleagues examined PI MZ subjects in two large populations: the GenKOLS case-control study and the family-based International COPD Genetics Network (ICGN) 30. In both studies, PI MZ heterozygotes had a reduced ratio of FEV 1 to forced vital capacity (FVC) compared to PI MM (3.5% in GenKOLS, 3.9% in ICGN). In GenKOLS, but not ICGN, PI MZ subjects had more emphysema on quantitative analysis of chest CT scans. There was no difference in COPD case-control status or in airway thickness on chest CT in either study.
Molloy et al. performed a family-based study in Ireland 6. They identified 51 PI MZ probands with COPD, and enrolled their family members. Comparing 99 PI MM and 89 PI MZ non-index relatives, PI MZ heterozygotes had reduced FEV 1 and FEV 1/FVC ratio compared to PI MM. In stratified analyses, this effect was strongest in ever-smokers and was not seen in never smokers. PI MZ was associated with an increased risk of COPD in all subjects (OR 5.18, 95% CI 1.27-21.15).
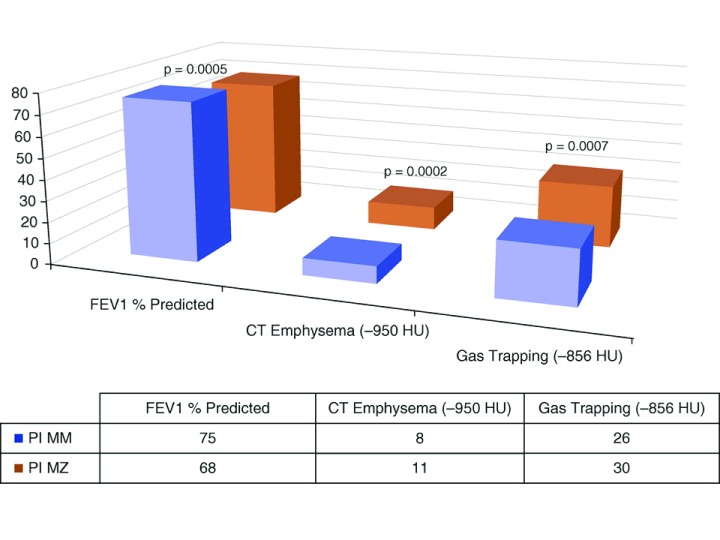
Within the COPDGene Study, a large U.S. cohort of current and former smokers with and without COPD, Foreman et al. performed genotyping for the SERPINA1 Z and S alleles 7. Out of over 8000 subjects, 261 PI MZ carriers were found, including 22 African Americans. Both white and African American PI MZ subjects had lower FEV 1 and FEV 1/FVC ratio compared to PI MM subjects. White PI MZ subjects had an increased risk of COPD (OR 1.42, 95% CI 1.05-1.93) and more emphysema on quantitative analysis of chest CT scans ( Figure 2). African American PI MZ subjects also had increased odds of COPD and increased CT emphysema, though the differences were not statistically significant, likely due to the small sample size.
Figure 2. Lung function and chest CT imaging in PI MZ subjects in the COPD Gene Study.
Non-Hispanic white subjects with PI MZ genotype had lower lung function and more emphysema and gas trapping on quantitative analysis of chest CT scans. FEV1 = forced expiratory volume in 1 second. HU = Hounsfield units. Reprinted from 7 with permission of the American Thoracic Society. Copyright © 2017 American Thoracic Society. Annals of the American Thoracic Society is an official journal of the American Thoracic Society.
In an abstract, Ortega et al. confirmed the association between PI MZ and reduced lung function and increased emphysema in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) 31. An exome genotyping array analysis of over 12,000 subjects also supported the association between PI MZ carriers and COPD 32. These studies have clearly demonstrated that PI MZ carriers are at an increased risk for airflow obstruction and COPD, especially in current and former smokers. Molloy et al. did not show an increased risk in never smokers 6; the other studies did not include enough never smokers to assess their risk.
The mechanisms for COPD risk in PI MZ subjects remain undetermined. Based on serum AAT levels in PI SS individuals, who are not at increased risk of COPD, and PI SZ individuals, who have an increased risk, a protective threshold of 11 μM (approximately 55mg/dl) has been established 9. However, serum AAT levels in PI MZ subjects remain above this threshold 33.
Although the emphysema in severe AAT deficiency has traditionally been attributed to the lack of inhibition of neutrophil elastase, there is increasing evidence that the Z protein itself may be deleterious in the lung. Accumulated Z-AAT produced in macrophages and lung epithelial cells leads to endoplasmic reticulum (ER) stress, which promotes inflammation, the unfolded protein response, and apoptosis 34. Z-AAT in the extracellular space may act as a neutrophil chemoattractant 35. A recent study of a Z-AAT transgenic mouse has supported the deleterious effect of the Z protein in the lungs 36. These basic mechanisms have been studied in the context of ZZ models, but the Z protein may also have local deleterious effects in the PI MZ lung.
Determining whether the increased COPD risk in PI MZ heterozygotes is due to low levels of AAT (loss of function) or due to the harmful effects of Z-AAT (toxic gain of function) could have therapeutic implications. In the former case, the concept of the protective threshold of 11 μM would have to be revisited, with perhaps different thresholds in current, former, and never smokers. In the latter, PI MZ heterozygotes could be candidates for clinical trials of novel treatments, such as compounds targeting misfolded proteins or ER stress. Regardless of mechanism, identifying PI MZ subjects has implications for risk assessment, family screening, and future precision medicine trials.
Clinical practice guidelines
The American Thoracic Society (ATS) first published clinical practice guidelines for the evaluation and treatment of patients with AAT deficiency in 1989 37. In collaboration with the European Respiratory Society (ERS), the ATS expanded and updated the guidelines in 2003 38. The Alpha-1 Foundation convened an expert panel, which reviewed the literature since 2002 and produced updated clinical practice guidelines, published in 2016 8.
The guidelines provide recommendations related to testing for AAT deficiency, evaluation of the patient with AAT deficiency, and treatment with intravenous augmentation therapy. The systematic review supports testing in all patients with COPD, as well as patients with other potential manifestations of AAT deficiency, specifically unexplained chronic liver disease, unexplained bronchiectasis, necrotizing panniculitis, and granulomatosis with polyangiitis. Testing is also recommended for first-degree relatives of subjects with severe AAT deficiency. In addition to protein levels, genotyping of at least the S and Z alleles is recommended for initial testing.
Two of the more controversial recommendations relate to evaluation of the newly diagnosed patient with AAT deficiency, namely full pulmonary function testing (spirometry, lung volumes and diffusing capacity) and a baseline chest CT scan 39. Chest CT scans are not currently recommended in the initial evaluation of patients with COPD unrelated to AAT deficiency 40, 41. The recommendations for serial spirometry to follow lung function and annual liver testing with clinical exam, ultrasound and laboratory studies are less controversial, although there is no clear consensus regarding the age to start liver evaluations 42. Serial chest CT scans are not recommended.
Indications for augmentation therapy are largely congruent with the RAPID trial described above. There is a recommendation for discussion of augmentation therapy in patients with milder lung disease (FEV 1 >65% predicted); declining diffusing capacity or emphysema progression on CT scan may help guide therapy in this situation. Augmentation therapy is not recommended in PI MZ carriers 43.
Conclusions
COPD is a major cause of morbidity and mortality worldwide. Severe AAT deficiency may be responsible for 1–2% of COPD cases in populations of European descent 44. Yet AAT deficiency remains under-diagnosed, due to inadequate awareness, lack of referral for appropriate testing, and possibly therapeutic nihilism regarding COPD in general. Three developments in the past several years should provide additional imperatives for diagnosis. Updated clinical practice guidelines provide clear recommendations regarding diagnosis and management 8. The RAPID trial has demonstrated the effectiveness of the augmentation therapy for AAT deficiency, with support for earlier initiation 5. This fits squarely into the modern paradigm of precision medicine, with a diagnostic test to identify a subtype of a heterogeneous disease, namely COPD, leading to a targeted treatment 45. And lastly, the demonstration of an increased risk of COPD in PI MZ carriers may have the broadest public health implications, since there may be over 6 million PI MZ carriers in the U.S. and over 27 million worldwide 46. Improved recognition of the role of AAT deficiency in lung and liver disease is crucial for the clinical care of these patients and necessary for the development of novel therapies.
Acknowledgements
The author thanks Drs. Dawn DeMeo and Edwin Silverman for their helpful comments.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Elizabeth Sapey, Institute of Inflammation and Ageing, Centre for Translational Inflammation Research, University of Birmingham, Birmingham, UK
Bibek Gooptu, Department of Molecular and Cell Biology, University of Leicester, Leicester, UK
Marc Miravitlles, Pneumology Department, Hospital Universitari Vall d'Hebron, Barcelona, Spain
Funding Statement
This work was funded by U.S. National Institutes of Health grants R01HL125583, R01HL130512, and P01HL105339.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Sveger T: Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med. 1976;294(24):1316–21. 10.1056/NEJM197606102942404 [DOI] [PubMed] [Google Scholar]
- 2. Silverman EK, Miletich JP, Pierce JA, et al. : Alpha-1-antitrypsin deficiency. High prevalence in the St. Louis area determined by direct population screening. Am Rev Respir Dis. 1989;140(4):961–6. 10.1164/ajrccm/140.4.961 [DOI] [PubMed] [Google Scholar]
- 3. Campos MA, Wanner A, Zhang G, et al. : Trends in the diagnosis of symptomatic patients with alpha1-antitrypsin deficiency between 1968 and 2003. Chest. 2005;128(3):1179–86. 10.1378/chest.128.3.1179 [DOI] [PubMed] [Google Scholar]
- 4. Stoller JK, Sandhaus RA, Turino G, et al. : Delay in diagnosis of alpha1-antitrypsin deficiency: a continuing problem. Chest. 2005;128(4):1989–94. 10.1378/chest.128.4.1989 [DOI] [PubMed] [Google Scholar]
- 5. Chapman KR, Burdon JG, Piitulainen E, et al. : Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9991):360–8. 10.1016/S0140-6736(15)60860-1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Molloy K, Hersh CP, Morris VB, et al. : Clarification of the risk of chronic obstructive pulmonary disease in α1-antitrypsin deficiency PiMZ heterozygotes. Am J Respir Crit Care Med. 2014;189(4):419–27. 10.1164/rccm.201311-1984OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foreman MG, Wilson C, DeMeo DL, et al. : Alpha-1 Antitrypsin PiMZ Genotype Is Associated with Chronic Obstructive Pulmonary Disease in Two Racial Groups. Ann Am Thorac Soc. 2017;14(8):1280–7. 10.1513/AnnalsATS.201611-838OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandhaus RA, Turino G, Brantly ML, et al. : The Diagnosis and Management of Alpha-1 Antitrypsin Deficiency in the Adult. Chronic Obstr Pulm Dis. 2016;3(3):668–82. 10.15326/jcopdf.3.3.2015.0182 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Silverman EK, Sandhaus RA: Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med. 2009;360(26):2749–57. 10.1056/NEJMcp0900449 [DOI] [PubMed] [Google Scholar]
- 10. Stoller JK, Aboussouan LS: A review of α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185(3):246–59. 10.1164/rccm.201108-1428CI [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Stockley RA: Alpha1-antitrypsin review. Clin Chest Med. 2014;35(1):39–50. 10.1016/j.ccm.2013.10.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Marciniak SJ, Ordóñez A, Dickens JA, et al. : New Concepts in Alpha-1 Antitrypsin Deficiency Disease Mechanisms. Ann Am Thorac Soc. 2016;13 Suppl 4:S289–96. 10.1513/AnnalsATS.201506-358KV [DOI] [PubMed] [Google Scholar]
- 13. Gadek JE, Klein HG, Holland PV, et al. : Replacement therapy of alpha 1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J Clin Invest. 1981;68(5):1158–65. 10.1172/JCI110360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wewers MD, Casolaro MA, Sellers SE, et al. : Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316(17):1055–62. 10.1056/NEJM198704233161704 [DOI] [PubMed] [Google Scholar]
- 15. Survival and FEV 1 decline in individuals with severe deficiency of alpha1-antitrypsin. The Alpha-1-Antitrypsin Deficiency Registry Study Group. Am J Respir Crit Care Med. 1998;158(1):49–59. 10.1164/ajrccm.158.1.9712017 [DOI] [PubMed] [Google Scholar]
- 16. Seersholm N, Wencker M, Banik N, et al. : Does alpha1-antitrypsin augmentation therapy slow the annual decline in FEV1 in patients with severe hereditary alpha1-antitrypsin deficiency? Wissenschaftliche Arbeitsgemeinschaft zur Therapie von Lungenerkrankungen (WATL) alpha1-AT study group. Eur Respir J. 1997;10(10):2260–3. [DOI] [PubMed] [Google Scholar]
- 17. Wencker M, Banik N, Buhl R, et al. : Long-term treatment of alpha1-antitrypsin deficiency-related pulmonary emphysema with human alpha1-antitrypsin. Wissenschaftliche Arbeitsgemeinschaft zur Therapie von Lungenerkrankungen (WATL)-alpha1-AT-study group. Eur Respir J. 1998;11(2):428–33. [DOI] [PubMed] [Google Scholar]
- 18. Dirksen A, Dijkman JH, Madsen F, et al. : A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1468–72. 10.1164/ajrccm.160.5.9901055 [DOI] [PubMed] [Google Scholar]
- 19. Dirksen A, Piitulainen E, Parr DG, et al. : Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J. 2009;33(6):1345–53. 10.1183/09031936.00159408 [DOI] [PubMed] [Google Scholar]
- 20. Stockley RA, Parr DG, Piitulainen E, et al. : Therapeutic efficacy of α-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res. 2010;11(1):136. 10.1186/1465-9921-11-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parr DG, Dirksen A, Piitulainen E, et al. : Exploring the optimum approach to the use of CT densitometry in a randomised placebo-controlled study of augmentation therapy in alpha 1-antitrypsin deficiency. Respir Res. 2009;10(1):75. 10.1186/1465-9921-10-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Washko GR: Diagnostic imaging in COPD. Semin Respir Crit Care Med. 2010;31(3):276–85. 10.1055/s-0030-1254068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hersh CP, Washko GR, Estépar RS, et al. : Paired inspiratory-expiratory chest CT scans to assess for small airways disease in COPD. Respir Res. 2013;14(1):42. 10.1186/1465-9921-14-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McElvaney NG, Burdon J, Holmes M, et al. : Long-term efficacy and safety of α1 proteinase inhibitor treatment for emphysema caused by severe α1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE). Lancet Respir Med. 2017;5(1):51–60. 10.1016/S2213-2600(16)30430-1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Sorrells S, Camprubi S, Griffin R, et al. : SPARTA clinical trial design: exploring the efficacy and safety of two dose regimens of alpha1-proteinase inhibitor augmentation therapy in alpha1-antitrypsin deficiency. Respir Med. 2015;109(4):490–9. 10.1016/j.rmed.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 26. Campos M, Lascano J: Therapeutics: Alpha-1 Antitrypsin Augmentation Therapy. Methods Mol Biol. 2017;1639:249–62. 10.1007/978-1-4939-7163-3_25 [DOI] [PubMed] [Google Scholar]
- 27. Loring HS, Flotte TR: Current status of gene therapy for α-1 antitrypsin deficiency. Expert Opin Biol Ther. 2015;15(3):329–36. 10.1517/14712598.2015.978854 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Laurell CB, Eriksson S: The electrophoretic α 1-globulin pattern of serum in α 1-antitrypsin deficiency. 1963. COPD. 2013;10 Suppl 1:3–8. 10.3109/15412555.2013.771956 [DOI] [PubMed] [Google Scholar]
- 29. Hersh CP, Dahl M, Ly NP, et al. : Chronic obstructive pulmonary disease in alpha1-antitrypsin PI MZ heterozygotes: a meta-analysis. Thorax. 2004;59(10):843–9. 10.1136/thx.2004.022541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sørheim IC, Bakke P, Gulsvik A, et al. : α 1-Antitrypsin protease inhibitor MZ heterozygosity is associated with airflow obstruction in two large cohorts. Chest. 2010;138(5):1125–32. 10.1378/chest.10-0746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ortega VE, Pasha S, Ampleford EJ, et al. : Rare SERPINA1 Variants are Associated with Lung Function and Emphysema in Non-Hispanic Whites from SPRIOMICS[abstract]. Am J Respir Crit Care Med. 2015;191:A3656. [Google Scholar]
- 32. Hobbs BD, Parker MM, Chen H, et al. : Exome Array Analysis Identifies a Common Variant in IL27 Associated with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2016;194(1):48–57. 10.1164/rccm.201510-2053OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferrarotti I, Thun GA, Zorzetto M, et al. : Serum levels and genotype distribution of α1-antitrypsin in the general population. Thorax. 2012;67(8):669–74. 10.1136/thoraxjnl-2011-201321 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Greene CM, McElvaney NG: Protein misfolding and obstructive lung disease. Proc Am Thorac Soc. 2010;7(6):346–55. 10.1513/pats.201002-019AW [DOI] [PubMed] [Google Scholar]
- 35. Mahadeva R, Atkinson C, Li Z, et al. : Polymers of Z alpha1-antitrypsin co-localize with neutrophils in emphysematous alveoli and are chemotactic in vivo. Am J Pathol. 2005;166(2):377–86. 10.1016/S0002-9440(10)62261-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alam S, Li Z, Atkinson C, et al. : Z α1-antitrypsin confers a proinflammatory phenotype that contributes to chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(8):909–31. 10.1164/rccm.201308-1458OC [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Guidelines for the approach to the patient with severe hereditary alpha-1-antitrypsin deficiency. American Thoracic Society. Am Rev Respir Dis. 1989;140(5):1494–7. 10.1164/ajrccm/140.5.1494 [DOI] [PubMed] [Google Scholar]
- 38. American Thoracic Society, European Respiratory Society: American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168(7):818–900. 10.1164/rccm.168.7.818 [DOI] [PubMed] [Google Scholar]
- 39. Stoller JK, Lacbawan FL, Aboussouan LS: Alpha-1 Antitrypsin Deficiency.In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews® [Internet]. Seattle WA: University of Washington, Seattle;2006. Reference Source [Google Scholar]
- 40. Vogelmeier CF, Criner GJ, Martínez FJ, et al. : Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch Bronconeumol. 2017;53(3):128–49. 10.1016/j.arbres.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 41. Yawn BB, Thomashaw B, Mannino DM, et al. : The 2017 Update to the COPD Foundation COPD Pocket Consultant Guide. Chronic Obstr Pulm Dis. 2017;4(3):177–85. 10.15326/jcopdf.4.3.2017.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Nelson DR, Teckman J, Di Bisceglie AM, et al. : Diagnosis and management of patients with α 1-antitrypsin (A1AT) deficiency. Clin Gastroenterol Hepatol. 2012;10(6):575–80. 10.1016/j.cgh.2011.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Sandhaus RA, Turino G, Stocks J, et al. : alpha1-Antitrypsin augmentation therapy for PI*MZ heterozygotes: a cautionary note. Chest. 2008;134(4):831–4. 10.1378/chest.08-0868 [DOI] [PubMed] [Google Scholar]
- 44. Lieberman J, Winter B, Sastre A: Alpha 1-antitrypsin Pi-types in 965 COPD patients. Chest. 1986;89(3):370–3. 10.1378/chest.89.3.370 [DOI] [PubMed] [Google Scholar]
- 45. Jameson JL, Longo DL: Precision medicine--personalized, problematic, and promising. N Engl J Med. 2015;372(23):2229–34. 10.1056/NEJMsb1503104 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. de Serres FJ: Worldwide racial and ethnic distribution of alpha1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest. 2002;122(5):1818–29. 10.1378/chest.122.5.1818 [DOI] [PubMed] [Google Scholar]