Key Points
Question
Does treatment with sertraline improve depressive symptoms in patients with stage 3, 4, or 5 non–dialysis-dependent chronic kidney disease and major depressive disorder?
Findings
In this randomized clinical trial that included 201 patients with non–dialysis-dependent chronic kidney disease and at least moderate depressive symptoms, the use of sertraline vs placebo did not result in a statistically significant difference in symptom improvement over 12 weeks (−4.1 points vs −4.2 points, respectively, of 27 possible points on the 16-item Quick Inventory of Depression Symptomatology–Clinician Rated).
Meaning
Sertraline may not be an effective treatment for major depressive disorder in patients with non–dialysis-dependent chronic kidney disease.
Abstract
Importance
Major depressive disorder (MDD) is prevalent among patients with chronic kidney disease (CKD) and is associated with morbidity and mortality. The efficacy and adverse events of selective serotonin reuptake inhibitors in these patients are unknown.
Objective
To determine whether treatment with sertraline improves depressive symptoms in patients with CKD and MDD.
Design, Setting, and Participants
The Chronic Kidney Disease Antidepressant Sertraline Trial (CAST) was a randomized, double-blind, placebo-controlled trial involving 201 patients with stage 3, 4, or 5 non–dialysis-dependent CKD, who were enrolled at 3 US medical centers. The Mini Neuropsychiatric Interview was used to establish MDD. The first participant was randomized in March 2010 and the last clinic visit occurred in November 2016.
Interventions
After a 1-week placebo run-in, participants were randomized to sertraline (n = 102) for 12 weeks at an initial dose of 50 mg/d (escalated to a maximum dose of 200 mg/d based on tolerability and response) or matching placebo (n = 99).
Main Outcomes and Measures
The primary outcome was improvement in depressive symptom severity from baseline to 12 weeks determined by the 16-item Quick Inventory of Depression Symptomatology–Clinician Rated (QIDS-C16) (score range, 0-27; minimal clinically important difference, 2 points). Secondary outcomes included improvement in quality of life (Kidney Disease Quality of Life Survey–Short Form; score range, 0-100; higher scores indicate more favorable quality of life) and adverse events.
Results
There were 201 patients (mean [SD] age, 58.2 [13.2] years; 27% female) randomized. The primary analysis included 193 patients who had at least 1 outcome assessment after randomization. The mean (SD) baseline QIDS-C16 score was 14.0 (2.4) in the sertraline group (n = 97) and 14.1 (2.4) in the placebo group (n = 96). The median participation time was 12.0 weeks and the median achieved dose was 150 mg/d, which was not significantly different between the groups. The QIDS-C16 score changed by −4.1 in the sertraline group and by −4.2 in the placebo group (between-group difference, 0.1 [95% CI, −1.1 to 1.3]; P = .82). There was no significant between-group difference in change in patient-reported overall health on the Kidney Disease Quality of Life Survey (median score, 0 in the sertraline group vs 0 in the placebo group; between-group difference, 0 [95% CI, −10.0 to 0]; P = .61). Nausea or vomiting occurred more frequently in the sertraline vs placebo group (22.7% vs 10.4%, respectively; between-group difference, 12.3% [95% CI, 1.9% to 22.6%], P = .03), as well as diarrhea (13.4% vs 3.1%; between-group difference, 10.3% [95% CI, 2.7% to 17.9%], P = .02).
Conclusions and Relevance
Among patients with non–dialysis-dependent CKD and MDD, treatment with sertraline compared with placebo for 12 weeks did not significantly improve depressive symptoms. These findings do not support the use of sertraline to treat MDD in patients with non–dialysis-dependent CKD.
Trial Registration
clinicaltrials.gov Identifier: NCT00946998
This randomized clinical trial compares the effects of sertraline vs placebo on depressive symptoms in patients with stage 3, 4, or 5 non–dialysis-dependent chronic kidney disease and major depressive disorder.
Introduction
Chronic kidney disease (CKD) affected 15% of the US population in 2016, and in 2013, depression was reported to be present in up to 25% of patients with CKD, which is 4 times higher than the prevalence in the general population. Comorbid depression was independently associated with hospitalization, cardiovascular events, and death among patients with non–dialysis-dependent CKD and among those dependent on dialysis. Depression also significantly worsened patient-centered outcomes and was associated with dialysis initiation, lower quality of life, greater symptom burden, sexual dysfunction, and nonadherence to medications and dietary restrictions.
Evidence for placebo-controlled efficacy of commonly used antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), is limited among patients with CKD, who generally have been excluded from large randomized trials of antidepressant medication treatment due to safety concerns. There is a need to establish whether antidepressant medications are safe and efficacious in patients with CKD who are at a disproportionately higher risk for both depression and its complications.
The purpose of this study was to determine whether treatment with sertraline would improve depressive symptoms in patients with non–dialysis-dependent stage 3, 4, or 5 CKD and major depressive disorder (MDD).
Methods
Study Design
The Chronic Kidney Disease Antidepressant Sertraline Trial (CAST) was a double-blind, placebo-controlled, parallel-design, 12-week flexible-dose randomized clinical trial conducted at 3 large medical centers in Dallas, Texas (University of Texas Southwestern Medical Center, Parkland Hospital, and the Veterans Affairs North Texas Health Care System). Detailed methods were published. The trial protocol appears in Supplement 1. The data analysis plan appears in Supplement 2.
The study was approved by the institutional review boards at each site and conducted per good clinical practice guidelines and the principles of the Declaration of Helsinki. Written informed consent was obtained from every participant prior to enrollment. The study was monitored by an independent data and safety monitoring board.
Participants and Eligibility
Individuals with stage 3, 4, or 5 non–dialysis-dependent CKD presenting to primary care or CKD clinics were screened with the 16-item Quick Inventory of Depressive Symptomatology–Self-Reported (QIDS-SR16) and deemed eligible if they had a score of 11 or greater (score range, 0-27). This instrument was previously validated for depression screening and shown to be independently associated with outcomes among individuals with CKD.
Individuals who provided consent completed the Mini International Neuropsychiatric Interview, which is based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV), to confirm current unipolar MDD and exclude other psychiatric conditions (ie, bipolar disorder or psychosis). Participants then completed a 1-week single-blind placebo run-in to exclude those who were nonadherent and ensure each individual still met the eligibility criterion of having a 16-item Quick Inventory of Depression Symptomatology–Clinician Rated (QIDS-C16) score of 11 or greater. Those who successfully completed the run-in (score of ≥11 on the QIDS-C16 and took ≥65% of study drug by pill count) were randomized by concealed allocation to 50 mg/d of either sertraline or matching placebo.
After randomization, participants attended clinic visits every 2 weeks for 6 weeks, then every 3 weeks for the remaining 6 weeks during the double-blind phase of the study (eFigure in Supplement 3). Dose was escalated by 50 mg/d at each successive visit to a potential maximum dose of 200 mg/d based on tolerability and response using a previously described measurement-based care protocol. The dose was kept constant for the last 6 weeks of the study. Adherence was ascertained by using pill counts at each visit, with nonadherence defined as having taken less than 80% of the drug. After 12 weeks, the drug was tapered at a rate of 50 mg/wk until completely discontinued. Participants were reassessed for depression 2 weeks after discontinuation and offered open-label sertraline or other therapy, which was managed by primary care or mental health physicians.
Adults with non–dialysis-dependent CKD stage 3, 4, or 5 (estimated glomerular filtration rate <60 mL/min/1.73 m2) were invited to participate. The inclusion criteria were changed to an estimated glomerular filtration rate of less than 45 mL/min/1.73 m2 early during the study in response to the National Institutes of Diabetes and Digestive and Kidney Diseases recommendation to reduce sample heterogeneity. There was no lower eligibility threshold for estimated glomerular filtration rate. Patients were excluded for the following reasons: inability to provide consent; having a functioning kidney transplant or requiring maintenance dialysis; having a transaminase elevation 3 or greater times the upper limit of normal; receiving current treatment with an antidepressant or other serotonergic drugs, or previous sertraline treatment failure; receiving psychotherapy for depression within 3 months; having psychosis or bipolar disorder, dementia, or suicidal intent; or being pregnant. Race and ethnicity were recorded by self-report based on fixed categories to describe the diversity of participants.
Randomization and Blinding
Patients were randomized in a 1:1 ratio using block randomization (block size range, 4-8) based on a computerized random-number generator, stratified by hospital site ([1] the University of Texas Southwestern Medical Center and Parkland Hospital or [2] the Veterans Affairs North Texas Health Care System) and CKD stage (3, 4, or 5). Treatment assignments were conducted by research pharmacists at each site using the prespecified random allocation sequence. Participants, researchers, and clinicians were blind to treatment assignment. The QIDS-C16 outcome measure was administered by trained personnel who were blind to treatment assignments and measurement-based care algorithms.
Study End Points
The prespecified primary outcome was improvement in depressive symptom severity from baseline to 12 weeks determined by the QIDS-C16 at baseline and weeks 2, 4, 6, 9, and 12. The QIDS-C16 assesses the 9 DSM-IV criterion symptoms of MDD, with higher scores (range, 0-27) indicating more severe depression.
Prespecified secondary outcomes included (1) response (≥50% decrease in baseline QIDS-C16 score) and remission (score decrease to ≤5); (2) quality-of-life end points and overall functioning assessed by the 5-item Work and Social Adjustment Scale (WSAS; in which each item is rated on a 0-8 Likert scale with 0 indicating no impairment; 8, severe impairment; score range, 0-40) and the Kidney Disease Quality of Life Survey–Short Form (KDQOL-SF version 1.3; score range, 0-100; higher scores signify more favorable quality of life), which were administered at baseline and at 6 and 12 weeks; (3) adverse events and tolerability assessed by the occurrence of serious adverse events (ie, death, dialysis initiation, hospitalizations, or bleeding requiring transfusion), maximal global adverse effects reported on the 56-item Systemic Assessment for Treatment Emergent Effects (SAFTEE; rated on a 0-4 ordinal scale with 0 indicating none; 4, marked adverse effects), and maximal frequency, intensity, and burden of adverse effects assessed with the Frequency, Intensity, and Burden of Side Effects Rating scale (each item rated on a Likert scale as present 0%, 10%, 25%, 50%, 75%, 90%, or all the time). Adverse events and tolerability were assessed at every visit.
Sample Size
Data collected during the pilot phase showed that individuals with CKD and MDD had a mean (SD) decrease in QIDS-SR16 score of 5.6 (4.1) points after treatment with an SSRI. A mean antidepressant drug-placebo difference of 3 points or greater on the Hamilton Rating Scale of Depression is recommended as a criterion for clinical significance when establishing guidelines for the treatment of depression. This is equivalent to a difference of 2 points on the QIDS-C16, which was regarded as the smallest clinically meaningful difference between the sertraline and placebo groups. Assuming a similar SD of 4 points yielded an effect size of 0.5, a t test comparison using a 2-sided α of .05 was estimated to be able to detect an effect size of 0.5 with 80% power in a sample of 128 participants (64 per group). A sample size of 200 (100 per group) was proposed so that if the SD was unexpectedly larger at 5 points, resulting in an effect size of 0.4, there would still be 80% power to detect a between-group difference.
Statistical Analysis
Using the modified intention-to-treat principle, participants were analyzed in the groups to which they were randomized if at least 1 QIDS-C16 assessment after randomization was available. The QIDS-C16 score was compared between groups using a repeated-measures mixed-effects model, with treatment group as the between-participants factor and random intercept and slopes as within-participant factors. Baseline QIDS-C16 was included as a covariate. The interactions between hospital site and CKD stage (3 vs 4 or 5) by treatment group and time were tested but not retained unless significant.
Response and remission were tested using the same procedure for a mixed-effects model, which is appropriate for a repeated binary outcome. A mixed-effects model for continuous outcomes as described for the primary analysis was used to compare changes in the WSAS and the KDQOL-SF, with baseline scores included as covariates. Given that (1) the amount of missing primary outcome data was small, (2) mixed-effects models allow inclusion of participants with missing data, and (3) these models are unbiased when data are missing at random, imputation of missing data was not done.
The proportion experiencing an adverse event; the maximum SAFTEE global assessment of adverse effects; and the maximal frequency, intensity, and burden of adverse effects at any visit were compared between groups using χ2 tests. A binary outcome (any adverse effect at any visit vs none) was created for each participant and compared between groups using the χ2 test. The mean number of worsening SAFTEE symptoms was compared between groups using a t test. Statistical tests were 2-sided and P < .05 was considered significant. The statistical analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
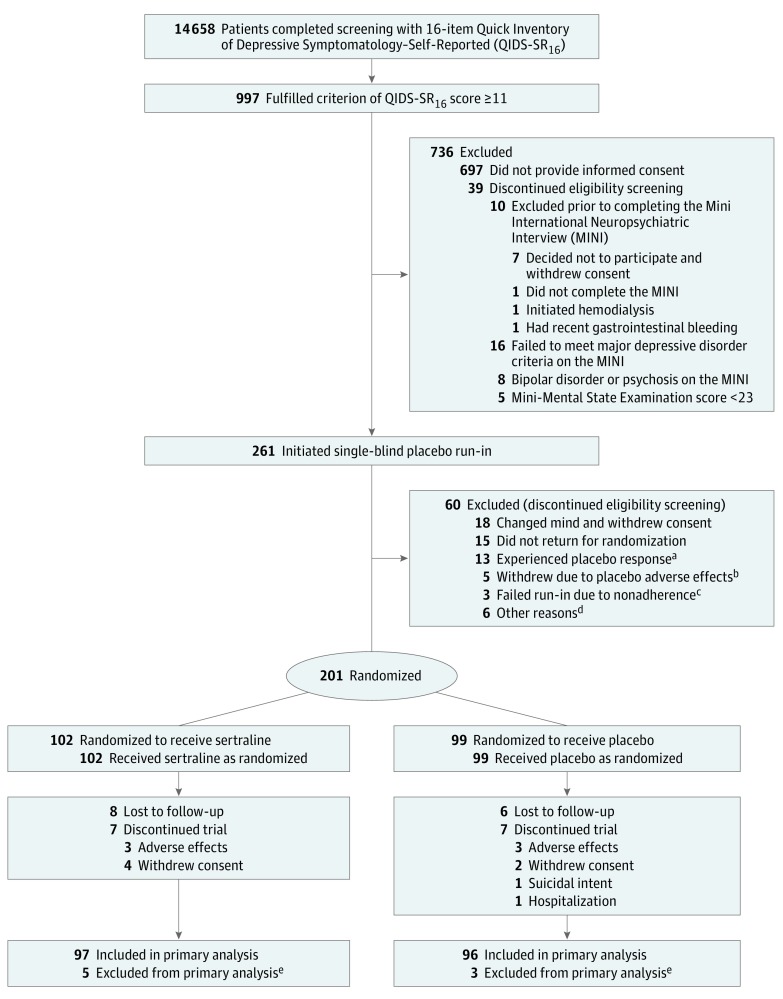
There were 201 patients (mean [SD] age, 58.2 [13.2] years; 27% female) randomized. The first participant was randomized in March 2010 and the last clinic visit occurred in November 2016. Of the 997 patients who qualified based on having a QIDS-SR16 score of 11 or greater and meeting other eligibility criteria, 697 did not provide consent. Of the 300 who provided consent, 261 met the Mini International Neuropsychiatric Interview MDD criteria and entered the placebo run-in. Sixty were excluded, leaving 201 to be randomized (Figure 1). Eight patients exited the study prior to the first QIDS-C16 assessment at week 2 and, therefore, had no outcome assessments after baseline and were excluded from the primary analysis. Therefore, 193 patients (97 in the sertraline group and 96 in the placebo group) constituted the modified intention-to-treat sample.
Figure 1. Patient Enrollment in the Chronic Kidney Disease Antidepressant Sertraline Trial (CAST).
aDefined as a 16-item Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C16) score of less than 11 after the placebo run-in phase.
bIncluded self-reported tremor, hypersomnolence, insomnia, diarrhea, and headache.
cDefined as having taken less than 65% of drug by pill count.
dEstimated glomerular filtration rate greater than 60 mL/min/1.73 m2 in 3 patients, ongoing substance dependence in 1 patient, suicidal ideation in 1 patient, and unable to obtain blood for laboratory tests in 1 patient.
eExited the trial prior to the first QIDS-C16 outcomes assessment after randomization (before week 2) and lacked any primary outcome data for analysis. Of the 5 patients in the sertraline group, 1 withdrew consent, 2 withdrew due to adverse effects, and 2 did not return for visits; of the 3 patients in the placebo group, 2 withdrew consent and 1 was hospitalized and no longer wanted to participate.
Baseline Characteristics
Of the 193 participants, 11% had stage 3A CKD; 36%, stage 3B; 36%, stage 4; and 17%, stage 5. The proportion in each CKD stage was not significantly different between treatment groups. Baseline characteristics were mostly balanced between groups, except that a larger proportion were receiving treatment with erythropoiesis-stimulating agents in the placebo group (7% vs 0% in the sertraline group; Table 1).
Table 1. Baseline Characteristics of Patients.
| No. (%) of Patientsa | ||
|---|---|---|
| Sertraline (n = 97) |
Placebo (n = 96) |
|
| Demographic Characteristics | ||
| Age, mean (SD), y | 57.7 (14.5) | 59.1 (12.2) |
| Male sex | 74 (76.3) | 67 (69.8) |
| Race | ||
| White | 41 (42.3) | 40 (41.7) |
| Black | 56 (57.7) | 55 (57.3) |
| Otherb | 0 | 1 (1.0) |
| Hispanic ethnicity | 21 (21.7) | 14 (14.6) |
| >High school education, No./total (%) | 73/96 (76.0) | 79/93 (85.0) |
| Married, No./total (%) | 33/93 (35.5) | 37/94 (39.4) |
| Lives alone, No./total (%) | 25/93 (26.9) | 25/94 (26.6) |
| Employed, No./total (%) | 20/92 (21.7) | 11/95 (11.6) |
| Medical Comorbidities | ||
| Chronic kidney disease (CKD) stagec | ||
| 3 | 46 (47.4) | 44 (45.8) |
| 4 | 34 (35.1) | 36 (37.5) |
| 5 | 17 (17.5) | 16 (16.7) |
| Charlson Comorbidity Index, mean (SD)d | 5.9 (2.3) | 6.3 (2.5) |
| Hypertension, No./total (%) | 94/96 (97.9) | 94/96 (97.9) |
| Diabetes mellitus, No./total (%) | 58/96 (60.4) | 55/96 (57.3) |
| Metabolic syndrome | 24 (24.7) | 28 (29.2) |
| Coronary artery disease, No./total (%) | 22/96 (22.9) | 21/95 (22.1) |
| Congestive heart failure, No./total (%) | 31/96 (32.3) | 31/96 (32.3) |
| Cerebrovascular disease, No./total (%) | 17/96 (17.7) | 13/96 (13.5) |
| Peripheral vascular disease, No./total (%) | 17/96 (17.7) | 10/94 (10.6) |
| Liver disease, No./total (%) | 9/95 (9.5) | 9/94 (9.6) |
| Lung disease, No./total (%) | 14/95 (14.7) | 14/94 (15.0) |
| Cancer, No./total (%) | 13/96 (13.5) | 15/95 (15.8) |
| Drug abuse, No./total (%) | 20/93 (21.5) | 23/96 (24.0) |
| Alcohol abuse, No./total (%) | 36/93 (38.7) | 43/96 (44.8) |
| Current tobacco use, No./total (%) | 20/92 (21.7) | 22/93 (23.7) |
| Concomitant Medications | ||
| Aspirin | 50 (51.6) | 42 (43.8) |
| Non–aspirin antiplatelet agent | 5 (5.2) | 9 (9.4) |
| Statin | 55 (56.7) | 60 (62.5) |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | 55 (56.7) | 43 (44.8) |
| Diuretic | 64 (66.0) | 66 (68.8) |
| β-Blocker | 53 (54.6) | 60 (62.5) |
| Calcium channel blocker | 56 (57.7) | 58 (60.4) |
| Hypnotic or sedative | 23 (23.7) | 19 (19.8) |
| Anticoagulant | 9 (9.3) | 7 (7.3) |
| Erythropoiesis-stimulating agent | 0 | 7 (7.3) |
| Laboratory Values | ||
| Estimated glomerular filtration rate, median (IQR), mL/min/1.73 m2 | 27.5 (18.0-35.0) | 27.5 (17.0-38.5) |
| Hemoglobin, mean (SD), g/dL | 11.9 (2.1) | 12.0 (2.2) |
| Creatinine, mean (SD), mg/dL | 3.2 (1.9) | 3.1 (1.9) |
| Blood urea nitrogen, mean (SD), mg/dL | 40.7 (18.9) | 41.1 (23.2) |
| Potassium, mean (SD), mEq/L | 4.4 (0.8) | 4.3 (0.7) |
| Carbon dioxide, mean (SD), mmol/L | 21.7 (4.9) | 22.1 (4.3) |
| Phosphorus, mean (SD), mg/dL | 4.1 (1.0) | 4.0 (0.9) |
| Calcium, mean (SD), mg/dL | 9.9 (8.8) | 10.1 (8.5) |
| Albumin, mean (SD), g/dL | 3.7 (0.6) | 3.9 (0.5) |
| Parathyroid hormone, median (IQR), g/mL | 126.0 (69.5-213.5) | 130.0 (78.0-223.0) |
| Total 25-hydroxyvitamin D, mean (SD), ng/mL | 26.2 (15.4) | 29.9 (14.3) |
| Cholesterol, mean (SD), mg/dL | ||
| Total | 170.7 (50.7) | 162.5 (39.9) |
| Low-density lipoprotein | 93.7 (47.2) | 86.1 (29.7) |
| High-density lipoprotein | 51.3 (20.1) | 47.8 (19.0) |
| Triglycerides, mean (SD), mg/dL | 137.9 (69.7) | 146.3 (100.3) |
| Hemoglobin A1C, median (IQR), % | 6.4 (5.8-7.6) | 6.2 (5.6-7.3) |
| C-reactive protein, median (IQR), mg/Le | 2.8 (1.1-7.2) | 3.3 (1.5-10.0) |
| IL-6, median (IQR), pg/mLe | 3.9 (2.3-7.8) | 3.8 (2.3-6.6) |
| Depression and Quality of Life | ||
| 16-Item Quick Inventory of Depressive Symptomatology-Clinician Rated score, mean (SD)f | ||
| All participants | 14.0 (2.4) | 14.1 (2.4) |
| CKD stage 3c | 13.9 (2.8) | 14.4 (2.7) |
| CKD stage 4c | 14.1 (2.0) | 13.9 (2.4) |
| CKD stage 5c | 13.9 (2.1) | 13.6 (2.0) |
| University of Texas Southwestern Medical Center and Parkland Hospital | 14.0 (2.2) | 14.0 (2.2) |
| Veterans Affairs North Texas Health Care System | 13.9 (2.6) | 14.1 (2.7) |
| Mini-Mental State Examination total score, mean (SD)g | 26.8 (2.2) | 26.5 (3.9) |
| Mini International Neuropsychiatric Interview psychiatric diagnoses, No./total (%) | ||
| Recurrent major depressive episode | 36/94 (38.3) | 41/94 (43.6) |
| Panic disorder | 1/93 (1.1) | 4/94 (4.3) |
| Agoraphobia | 9/93 (9.7) | 9/94 (9.6) |
| Social phobia | 4/93 (4.3) | 9/94 (9.6) |
| Obsessive compulsive disorder | 3/93 (3.2) | 4/94 (4.3) |
| Posttraumatic stress disorder | 6/93 (6.5) | 11/94 (11.7) |
| Generalized anxiety disorder | 29/93 (31.2) | 27/94 (28.7) |
| Antisocial personality disorder | 0 | 1/93 (1.1) |
| Alcohol dependence | 1/93 (1.1) | 2/94 (2.1) |
| Alcohol abuse | 2/93 (2.2) | 1/94 (1.1) |
| Substance dependence | 6/93 (6.5) | 1/94 (1.1) |
| Substance abuse | 3/93 (3.2) | 0 |
| 5-Item Work and Social Adjustment Scale total score, mean (SD)h | 20.4 (11.0) | 18.7 (11.5) |
| Kidney Disease Quality of Life Survey–Short Form score, median (IQR)i | ||
| Kidney disease component | ||
| Symptoms and problems | 68.2 (58.3-77.3) | 68.2 (56.8-77.3) |
| Adverse effects | 65.6 (43.8-81.3) | 68.8 (50.0-78.1) |
| Burden | 43.8 (25.0-62.5) | 37.5 (25.0-56.3) |
| Work status | 0 (0-50.0) | 0 (0-50.0) |
| Cognitive function | 66.7 (53.3-80.0) | 66.7 (46.7-80.0) |
| Quality of social interaction | 60.0 (53.3-73.3) | 60.0 (46.7-73.3) |
| Sexual function | 56.3 (25.0-75.0) | 68.8 (31.3-100.0) |
| Sleep | 50.0 (40.0-62.5) | 52.5 (40.0-65.0) |
| Social support | 66.7 (50.0-83.3) | 50.0 (33.3-83.3) |
| Patient satisfaction | 66.7 (50.0-83.3) | 66.7 (50.0-83.3) |
| Physical functioning component | ||
| Functioning | 35.0 (15.0-60.0) | 25.0 (15.0-50.0) |
| Role limits | 0 (0-25.0) | 0 (0-25.0) |
| Pain | 45.0 (22.5-67.5) | 40.0 (22.5-57.5) |
| General health | 30.0 (20.0-45.0) | 30.0 (20.0-40.0) |
| Mental functioning component | ||
| Emotional well-being | 60.0 (44.0-68.0) | 56.0 (44.0-68.0) |
| Emotional role limits | 0 (0-66.7) | 0 (0-33.3) |
| Social function | 50.0 (37.5-62.5) | 37.5 (25.0-62.5) |
| Energy | 35.0 (20.0-50.0) | 30.0 (20.0-45.0) |
| Overall health | 50.0 (30.0-50.0) | 50.0 (30.0-50.0) |
| 12-Item short form | ||
| Physical health composite | 29.1 (25.8-36.6) | 28.6 (24.7-36.9) |
| Mental health composite | 38.2 (33.0-46.1) | 38.0 (32.3-43.0) |
Abbreviation: IQR, interquartile range.
SI conversion factors: To convert calcium to mmol/L, multiply by 0.25; C-reactive protein to nmol/L, multiply by 9.524; creatinine to μmol/L, multiply by 88.4; high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and total cholesterol to mmol/L, multiply by 0.0259; phosphorus to mmol/L, multiply by 0.323; triglycerides to mmol/L, multiply by 0.0113; total 25-hydroxyvitamin D to nmol/L, multiply by 2.496.
Unless otherwise indicated.
Included Asian, American Indian, Alaska Native, Native Hawaiian, and Pacific Islander.
Stage 3 defined as estimated glomerular filtration rate of 30 to 59 mL/min/1.73 m2; stage 4, 15 to 29 mL/min/1.73 m2; stage 5, less than 15 mL/min/1.73 m2.
Score range of 0 to 37; higher scores indicate greater burden of comorbidities.
Measured by high-sensitivity assays.
Score range of 0 to 27; higher scores indicate more severe depression; a score of 0 to 5 corresponds to a normal affect; 6 to 10 to a mild affect; 11 to 15 to a moderate affect; 16 to 20 to a severe affect; and 21 or greater to very severe affect.
Second edition; score range of 0 to 30 with scores of 0 to 17 indicating severe cognitive impairment; 18 to 23, mild impairment; and 24 to 30, no impairment.
Each item is rated on a 0 to 8 Likert scale with 0 indicating no impairment and 8 indicating severe impairment and a total score range of 0 to 40.
Raw scores from version 1.3 were transformed to a scale from 0 to 100, in which higher numbers signify more favorable quality of life.
The mean (SD) baseline score on the QIDS-C16 (minimum score, 0; maximum, 27) was 14.0 (2.4) in the sertraline group and 14.1 (2.4) in the placebo group. There were no clinically meaningful between-group differences in the proportion with comorbid psychiatric illnesses, in the Mini-Mental State Examination scores, or in the quality-of-life scores (Table 1). Six participants in the sertraline group had a history of substance dependence compared with 1 participant in the placebo group.
Treatment Characteristics
The median treatment duration was 84.0 days (interquartile range, 83.0 to 87.0 days; between-group difference, 0 days [95% CI, 0 to 2.0 days], P = .19; Table 2). Altogether, 92% completed at least 6 weeks and 84% completed all 12 weeks of the study. The number of postrandomization visits and the proportion that completed 4, 6, 9, or 12 weeks of the study were not significantly different between the groups (Table 2). The median achieved sertraline dose was 150 mg/d (interquartile range, 100 to 150 mg/d; between-group difference, 0 mg/d [95% CI, −50.0 to 0 mg/d], P = .10). The mean percentage of drug taken ascertained by pill count was 94% in the sertraline group and 96% in the placebo group (between-group difference, −1.6% [95% CI, −4.7% to 1.4%], P = .29). Three participants in the sertraline group and 2 in the placebo group were nonadherent (P = .66).
Table 2. Characteristics of Treatment.
| No. (%) of Patientsa | Between-Group Difference, % (95% CI)a |
P Value |
||
|---|---|---|---|---|
| Sertraline (n = 97) | Placebo (n = 96) | |||
| Duration of treatment | ||||
| Total, median (IQR), d | 84.0 (84.0 to 87.0) | 84.0 (82.5 to 86.0) | 0 (0 to 2.0) | .19b |
| 4 wk | 91 (93.8) | 93 (96.9) | −3.1 (−9.0 to 2.9) | .31c |
| 6 wk | 89 (91.8) | 88 (91.7) | 0.1 (−7.7 to 7.9) | .98c |
| 9 wk | 84 (86.6) | 83 (86.5) | 0.1 (−9.5 to 9.8) | .98c |
| 12 wk | 81 (83.5) | 81 (84.4) | −0.9 (−11.2 to 9.5) | .87c |
| No. of visits after randomization, median (IQR) | 6 (6 to 6) | 6 (6 to 6) | 0 (0 to 0) | .52b |
| Drug dose, mg/d | ||||
| At study exit, median (IQR) | 150 (100 to 150) | 150 (100 to 200) | 0 (−50 to 0) | .10b |
| 0d | 2 (2.1) | 1 (1.0) | 1.0 (−2.5 to 4.5) | .20c |
| 50 | 19 (19.6) | 9 (9.4) | 10.2 (0.4 to 20.0) | |
| 100 | 20 (20.6) | 25 (26.0) | −5.4 (−17.3 to 6.5) | |
| 150 | 37 (38.1) | 34 (35.4) | 2.7 (−10.9 to 16.3) | |
| 200 | 19 (19.6) | 27 (28.1) | −8.5 (−20.5 to 3.4) | |
| Medication adherence | ||||
| Total drug taken, mean (95% CI), %e | 94.4 (91.7 to 97.0) | 96.0 (94.5 to 97.5) | −1.6 (−4.7 to 1.4) | .29f |
| Nonadherentg | 3 (3.1) | 2 (2.1) | 1.0 (−3.5 to 5.5) | >.99h |
Abbreviation: IQR, interquartile range.
Unless otherwise indicated.
Based on Wilcoxon 2-sample test.
Based on the χ2 test.
Discontinued treatment at 12 weeks.
Measured at each visit after randomization and was determined by pill count.
Based on the t test.
Any instance of taking less than 80% of study drug based on pill count at each visit after randomization.
Based on the Fisher exact test.
Efficacy Outcomes
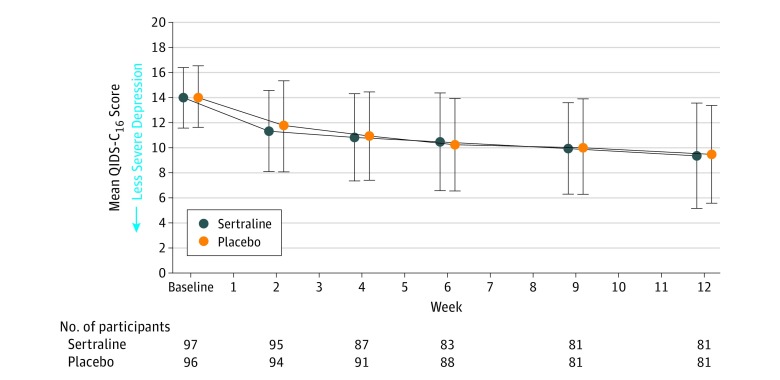
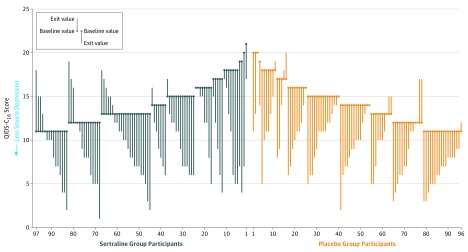
The mixed-effects model showed no significant treatment group main effect (P = .82) or interaction with time (P = .71) in the QIDS-C16 scores (Figure 2). The mean change from baseline to study exit in the QIDS-C16 score was −4.1 in the sertraline group and −4.2 in the placebo group (between-group difference, 0.1 [95% CI, −1.1 to 1.3], P = .82; Table 3). Figure 3 illustrates changes in the QIDS-C16 score from baseline to study exit for individual participants.
Figure 2. Serial Changes in the 16-Item Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C16) Scores.
Participants completed at least 1 assessment after randomization and were included in the primary analysis. Error bars indicate SDs, which were calculated separately for each time point. Each of the 16 QIDS-C16 items can yield a score of 0 to 3 on a Likert scale. The score range is 0 to 27; higher scores indicate more severe depression; a score of 0 to 5 corresponds to a normal affect; 6 to 10 to a mild affect; 11 to 15 to a moderate affect; 16 to 20 to a severe affect; and 21 or greater to very severe depression.
Table 3. Treatment Efficacy End Points.
| Mean Change From Baseline to Study Exit or Wk 12a | Between-Group Difference, Mean (95% CI)a |
P Value |
||
|---|---|---|---|---|
| Sertraline (n = 97) |
Placebo (n = 96) |
|||
| Primary End Point | ||||
| QIDS-C16 score in total cohort, mean (95% CI)b | −4.1 (−5.1 to −3.1) | −4.2 (−5.0 to −3.5) | 0.1 (−1.1 to 1.3) | .82c |
| Secondary End Points | ||||
| QIDS-C16 score, mean (95% CI)b | ||||
| Chronic kidney disease stage 3d | −4.3 (−5.9 to −2.7) | −5.0 (−6.1 to −3.9) | 0.7 (−1.2 to 2.6) | .47c |
| Chronic kidney disease stage 4e | −4.3 (−5.8 to −2.9) | −3.8 (−4.8 to −2.7) | −0.5 (−2.3 to 1.2) | .54c |
| Chronic kidney disease stage 5f | −3.1 (−5.4 to −0.8) | −3.1 (−5.3 to −1.0) | 0.1 (−3.0 to 3.1) | .97c |
| University of Texas Southwestern Medical Center and Parkland Hospital | −3.7 (−4.9 to −2.4) | −3.1 (−4.1 to −2.1) | −0.6 (−2.2 to 1.0) | .47c |
| Veterans Affairs North Texas Health Care System | −4.5 (−6.1 to −3.0) | −5.4 (−6.3 to −4.5) | 0.9 (−0.9 to 2.6) | .33c |
| Response assessed by QIDS-C16 score, No./total (%)g | ||||
| Total cohort | 31/97 (32.0) | 24/96 (25.0) | 7.0 (−5.7 to 19.6) | .28h |
| Chronic kidney disease stage 3d | 17/46 (37.0) | 14/44 (31.8) | 5.1 (−14.5 to 24.7) | .61h |
| Chronic kidney disease stage 4e | 10/34 (29.4) | 6/36 (16.7) | 12.8 (−6.8 to 32.3) | .20h |
| Chronic kidney disease stage 5f | 4/17 (23.5) | 4/16 (25.0) | −1.5 (−30.7 to 27.8) | .99i |
| University of Texas Southwestern Medical Center and Parkland Hospital | 12/48 (25.0) | 9/48 (18.8) | 6.2 (−10.2 to 22.7) | .46h |
| Veterans Affairs North Texas Health Care System | 19/49 (38.8) | 15/48 (31.3) | 7.5 (−11.4 to 26.4) | .44h |
| Remission assessed by QIDS-C16 score, No./total (%)j | ||||
| Total cohort | 15/97 (15.5) | 14/96 (14.6) | 0.9 (−9.2 to 11.0) | .86h |
| Chronic kidney disease stage 3d | 10/46 (21.7) | 9/44 (20.5) | 1.3 (−15.6 to 18.1) | .88h |
| Chronic kidney disease stage 4e | 4/34 (11.8) | 2/36 (5.6) | 6.2 (−7.0 to 19.4) | .42i |
| Chronic kidney disease stage 5f | 1/17 (5.9) | 3/16 (18.8) | −12.9 (−35.0 to 9.3) | .34i |
| University of Texas Southwestern Medical Center and Parkland Hospital | 5/48 (10.4) | 5/48 (10.4) | 0 (−12.2 to 12.2) | >.99h |
| Veterans Affairs North Texas Health Care System | 10/49 (20.4) | 9/48 (18.8) | 1.7 (−14.1 to 17.4) | .84h |
| Work and Social Adjustment Scale score for overall functioning, mean (95% CI)k | −5.0 (−7.6 to −2.5) | −3.2 (−5.8 to −0.7) | −1.8 (−5.4 to 1.8) | .32c |
| Kidney Disease Quality of Life Survey–Short Form score, median (IQR)l,m | ||||
| Kidney disease component | ||||
| Symptoms and problems | 9.1 (2.3 to 15.9) | 10.0 (0 to 18.2) | −0.6 (−4.5 to 3.2) | .62 |
| Adverse effects | 9.4 (−3.1 to 21.9) | 3.1 (−6.3 to 18.8) | 3.1 (−3.1 to 9.4) | .25 |
| Burden | 6.3 (−6.3 to 25.0) | 6.3 (−6.3 to 25.0) | 0 (−6.2 to 6.2) | .62 |
| Work status | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | .45 |
| Cognitive function | 6.7 (0 to 20.0) | 6.7 (0 to 20.0) | 0 (−6.7 to 6.7) | .85 |
| Quality of social interaction | 6.7 (−6.7 to 20.0) | 13.3 (0 to 20.0) | 0 (−6.7 to 0) | .31 |
| Sexual function | 12.5 (0 to 25.0) | 12.5 (0 to 37.5) | 0 (−12.5 to 12.5) | .97 |
| Sleep | 12.5 (0 to 25.0) | 7.5 (−5.0 to 18.8) | 5.0 (0 to 12.5) | .03 |
| Social support | 0 (0 to 16.7) | 0 (−16.7 to 16.7) | 0 (0 to 16.7) | .34 |
| Patient satisfaction | 0 (0 to 16.7) | 0 (−16.7 to 16.7) | 0 (0 to 16.7) | .97 |
| Physical functioning component | ||||
| Functioning | 5.0 (−10.0 to 16.1) | 0 (−5.0 to 15.0) | 0 (−5.0 to 5.0) | .89 |
| Role limits | 0 (0 to 25.0) | 0 (0 to 25.0) | 0 (0 to 25.0) | .19 |
| Pain | 10.0 (0 to 22.5) | 10.0 (0 to 22.5) | 0 (−7.5 to 7.5) | .95 |
| General health | 5.0 (−5.0 to 15.0) | 3.5 (−5.0 to 10.0) | 0 (−5.0 to 5.0) | .76 |
| Mental functioning component | ||||
| Emotional well-being | 12.0 (−4.0 to 24.0) | 8.0 (0 to 24.0) | 0 (−8.0 to 4.0) | .85 |
| Emotional role limits | 0 (0 to 66.7) | 0 (0 to 33.3) | 0 (0 to 0) | .64 |
| Social function | 12.5 (−12.5 to 25.0) | 12.5 (0 to 25.0) | 0 (−12.5 to 12.5) | .64 |
| Energy | 5.0 (−5.0 to 25.0) | 10.0 (0 to 20.0) | −5.0 (−10.0 to 5.0) | .35 |
| Overall health | 0 (−10.0 to 10.0) | 0 (0 to 10.0) | 0 (−10.0 to 0) | .61 |
| 12-Item short form | ||||
| Physical health composite | 1.8 (−3.5 to 6.8) | 0.5 (−3.1 to 5.5) | 0.9 (−1.3 to 3.2) | .41 |
| Mental health composite | 4.9 (−1.8 to 14.8) | 4.5 (−1.1 to 13.5) | −0.8 (−4.1 to 2.6) | .63 |
Abbreviations: IQR, interquartile range; QIDS-C16, 16-Item Quick Inventory of Depressive Symptomatology-Clinician Rated.
Unless otherwise indicated.
Scores range from 0 to 27; higher scores indicate a greater severity of depressive symptoms.
Based on the t test.
Defined as estimated glomerular filtration rate of 30 to 59 mL/min/1.73 m2.
Defined as estimated glomerular filtration rate of 15 to 29 mL/min/1.73 m2.
Defined as estimated glomerular filtration rate of less than 15 mL/min/1.73 m2.
Defined as a decrease of 50% or greater from baseline.
Based on the χ2 test.
Based on the Fisher exact test.
Defined as a score of 5 or less at study exit.
Each item is rated on a 0 to 8 Likert scale with 0 indicating no impairment and 8 indicating severe impairment and a total score range of 0 to 40.
Raw scores from version 1.3 were transformed to a scale from 0 to 100, in which higher numbers signify more favorable quality of life.
Based on Wilcoxon 2-sample test.
Figure 3. Paired Baseline and End of Study 16-Item Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C16) Scores.
Parallel line plot of the QIDS-C16 scores. Each patient is represented by a vertical line, with participants plotted by group assignment (sertraline, n = 97; placebo, n = 96) and sorted by baseline value.
The binary mixed-effects model for remission showed no treatment group main effect (P = .57) or interaction with time (P = .58). The proportion with remission was 15.5% in the sertraline group and 14.6% in the placebo group (between-group difference, 0.9% [95% CI, −9.2% to 11.0%], P = .86; Table 3). The mixed-effects model for response showed no treatment group main effect (P = .54) or interaction with time (P = .97). The proportion of participants with a treatment response was 32.0% in the sertraline group and 25.0% in the placebo group (between-group difference, 7.0% [95% CI, −5.7% to 19.6%], P = .28; Table 3).
Functioning and Quality-of-Life Outcomes
The mixed-effects model for the WSAS showed no significant treatment group main effect (P = .64) or interaction with time (P = .33). The mean change from baseline to study exit in overall functioning by WSAS score was not significantly different (between-group mean difference, −1.8 [95% CI, −5.4 to 1.8], P = .32; Table 3).
There was no significant difference in change in patient-reported overall health on the KDQOL-SF (median, 0 vs 0; between-group median difference, 0 [95% CI, −10.0 to 0]; P = .61). The mixed-effects model for the physical component of the KDQOL-SF also found no significant treatment group main effect (P = .55) or interaction with time (P = .98). No differences were observed in quality-of-life components from baseline to study exit, except for improvement in sleep, which was significantly greater in those receiving sertraline (between-group median difference, 5.0 [95% CI, 0 to 12.5], P = .03; Table 3). The 12-week or study exit efficacy and quality-of-life measures for each treatment group are included in the eTable in Supplement 3.
Adverse Events and Tolerability Outcomes
The groups did not significantly differ in the proportion that experienced 1 or more prespecified serious adverse events (Table 4). One participant receiving placebo developed acute suicidal intent; however, there were no suicides. Another participant receiving 200 mg/d of sertraline was hospitalized with elevated liver enzyme levels. Liver biopsy indicated drug-induced acute liver injury, and sertraline was discontinued. Between randomization and the first assessment of outcomes, 2 participants assigned to sertraline withdrew due to adverse effects (Figure 1).
Table 4. Adverse Events and Tolerability.
| No. of Patients/Total (%)a | Between-Group Difference, % (95% CI)a |
P Value |
||
|---|---|---|---|---|
| Sertraline | Placebo | |||
| Prespecified serious adverse eventsb,c | ||||
| Death during study period | 0/97 | 0/96 | 0 (0 to 0) | >.99 |
| Dialysis initiation | 7/97 (7.2) | 5/96 (5.2) | 2.0 (−4.8 to 8.8) | .77 |
| Hospitalization other than for dialysis initiation | 8/97 (8.2) | 7/96 (7.3) | 1.0 (−6.6 to 8.5) | >.99 |
| Bleeding episode requiring blood transfusion or hospitalization | 2/97 (2.1) | 2/96 (2.1) | 0 (−4.0 to 4.0) | >.99 |
| Acute suicidal intent | 0/97 | 1/96 (1.0) | −1.0 (−3.1 to 1.0) | .50 |
| Other serious adverse events | ||||
| Stroke | 1/97 (1.0) | 1/96 (1.0) | 0 (−2.9 to 2.8) | >.99 |
| Acute myocardial infarction | 0/97 | 1/96 (1.0) | −1.0 (−3.1 to 1.0) | .50 |
| Hospital admission for congestive heart failure | 2/97 (2.1) | 2/96 (2.1) | 0 (−4.0 to 4.0) | >.99 |
| Drug discontinuation due to intolerancec,d | 3/97 (3.1) | 3/96 (3.1) | 0 (−4.9 to 4.9) | >.99 |
| Systemic Assessment for Treatment Emergent Effects Scale | ||||
| 56-Item maximal global assessmente | ||||
| None | 11/91 (12.1) | 12/94 (12.8) | −0.7 (−10.1 to 8.8) | .92f |
| Mild | 37/91 (40.7) | 35/94 (37.2) | 3.4 (−10.6 to 17.5) | |
| Moderate | 30/91 (33.0) | 35/94 (37.2) | −4.3 (−18.0 to 9.5) | |
| Marked | 13/91 (14.3) | 12/94 (12.8) | 1.5 (−8.3 to 11.4) | |
| Worsened condition, mean (95% CI)g | 18.8 (16.5 to 21.2) | 17.7 (15.9 to 19.5) | 1.1 (−1.8 to 4.0) | .44h |
| Frequency, Intensity, and Burden of Side Effects Rating Scalei | ||||
| Maximal frequency, % | ||||
| 0 | 22/92 (23.9) | 38/95 (40.0) | −16.1 (−29.2 to −2.9) | .13f |
| 10-25 | 33/92 (35.9) | 27/95 (28.4) | 7.4 (−5.9 to 20.8) | |
| 50-75 | 24/92 (26.1) | 20/95 (21.1) | 5.0 (−7.1 to 17.2) | |
| 90-100 | 13/92 (14.1) | 10/95 (10.5) | 3.6 (−5.8 to 13.0) | |
| Any (vs none) | 70/92 (76.1) | 57/95 (60.0) | 16.1 (2.9 to 29.2) | .02f |
| Maximal intensity | ||||
| None | 19/92 (20.7) | 32/95 (33.7) | −13.0 (−25.6 to −0.4) | .13f |
| Minimal to mild | 25/92 (27.2) | 28/95 (29.5) | −2.3 (−15.2 to 10.6) | |
| Moderate to marked | 35/92 (38.0) | 27/95 (28.4) | 9.6 (−3.8 to 23.1) | |
| Severe to intolerable | 13/92 (14.1) | 8/95 (8.4) | 5.7 (−3.3 to 14.8) | |
| Any (vs none) | 73/92 (79.3) | 63/95 (66.3) | 13.0 (0.4 to 25.6) | .04f |
| Maximal burden | ||||
| None | 28/92 (30.4) | 38/95 (40.0) | −9.6 (−23.2 to 4.0) | .45f |
| Minimal to mild | 35/92 (38.0) | 27/95 (28.4) | 9.6 (−3.8 to 23.1) | |
| Moderate to marked | 22/92 (23.9) | 24/95 (25.3) | −1.4 (−13.7 to 11.0) | |
| Severe to intolerable | 7/92 (7.6) | 6/95 (6.3) | 1.3 (−6.0 to 8.6) | |
| Any (vs none) | 64/92 (69.6) | 57/95 (60.0) | 9.6 (−4.0 to 23.2) | .17f |
| Type of adverse event during 12 wks of study treatmentb,c | ||||
| Nausea, indigestion, or vomiting | 22/97 (22.7) | 10/96 (10.4) | 12.3 (1.9 to 22.6) | .03 |
| Diarrhea | 13/97 (13.4) | 3/96 (3.1) | 10.3 (2.7 to 17.9) | .02 |
| Anorexia | 1/97 (1.0) | 1/96 (1.0) | 0 (−2.9 to 2.8) | >.99 |
| Increased appetite | 1/97 (1.0) | 2/96 (2.1) | −1.0 (−4.6 to 2.4) | .62 |
| Insomnia | 7/97 (7.2) | 10/96 (10.4) | −3.2 (−11.2 to 4.8) | .46 |
| Somnolence | 9/97 (9.3) | 5/96 (5.2) | 4.1 (−3.2 to 11.4) | .41 |
| Decreased memory or confusion | 2/97 (2.1) | 3/96 (3.1) | −1.0 (−5.6 to 3.4) | .68 |
| Incident hyponatremia | 3/78 (3.8) | 2/74 (2.7) | 1.1 (−4.5 to 6.8) | .69 |
| Dizziness, syncope, or fall | 8/97 (8.3) | 4/96 (4.2) | 4.1 (−2.7 to 10.9) | .37 |
| Anxiety, irritability, or agitation | 7/97 (7.2) | 4/96 (4.2) | 3.0 (−3.5 to 9.6) | .54 |
| Tremor | 3/97 (3.1) | 1/96 (1.0) | 2.0 (−2.0 to 6.0) | .62 |
| Headache | 6/97 (6.2) | 4/96 (4.2) | 2.0 (−4.2 to 8.3) | .75 |
| Dry mouth | 4/97 (4.1) | 4/96 (4.2) | 0 (−5.7 to 5.6) | >.99 |
| Rash | 3/97 (3.1) | 1/96 (1.0) | 2.0 (−2.0 to 6.0) | .62 |
| Decreased libido or erectile dysfunction | 3/97 (3.1) | 2/96 (2.1) | 1.0 (−3.5 to 5.5) | >.99 |
Unless otherwise indicated.
Participants may have had more than 1 event.
Based on the Fisher exact test.
Includes if the participant cited intolerable adverse effects as a reason for stopping use of the study drug.
Participant was instructed to rate all symptoms and indicate whether due to study medication. Each item was given a score from 0 to 4 on an ordinal scale.
Based on the χ2 test.
Number of symptoms that worsened at any point during the study for each participant.
Based on the t test.
Participant was instructed to rate only adverse effects of study medication. Frequency, intensity, and burden of adverse effects were rated on a 6-point Likert scale.
Of the 193 participants included in the modified intention-to-treat analysis, 3 (3.1%) assigned to sertraline and 3 (3.1%) to placebo discontinued treatment due to drug intolerance (between-group difference, 0% [95% CI, −4.9% to 4.9%], P > .99) (Table 4). Although there were no statistically significant differences for worsening maximal adverse effect frequency, intensity, or burden, the proportion with any vs no adverse effects was higher in the sertraline group (76.1%) compared with the placebo group (60.0%; between-group difference, 16.1% [95% CI, 2.9% to 29.2%], P = .02). Nausea or vomiting was reported by 22.7% of the sertraline group compared with 10.4% of the placebo group (between-group difference, 12.3% [95% CI, 1.9% to 22.6%], P = .03) and diarrhea by 13.4% vs 3.1%, respectively (between-group difference, 10.3% [95% CI, 2.7% to 17.9%], P = .02; Table 4).
Discussion
Treatment with sertraline did not improve depressive symptoms or quality of life in patients with stage 3, 4, or 5 non–dialysis-dependent CKD and resulted in increased adverse effects compared with placebo. To our knowledge, this is the largest randomized, double-blind, placebo-controlled trial to provide evidence about MDD treatment with a commonly used SSRI in patients with non–dialysis-dependent CKD, a chronically ill population that is not only at significantly increased risk for developing depression, but also its serious complications.
Although the risk of serious adverse events was not higher in patients receiving sertraline vs placebo, those treated with sertraline did experience a significantly higher incidence of gastrointestinal adverse effects. These results provide evidence regarding the lack of efficacy of sertraline among patients with non–dialysis-dependent CKD that would change clinical practice.
Previous studies investigating the efficacy of antidepressant medications among patients with CKD were performed in dialysis-dependent samples and were limited by lack of randomization and control, small sample sizes, short treatment durations, low adherence, high dropout rates, and nonstandard criteria for MDD diagnosis. Only 2 trials appropriately diagnosed MDD by using a psychiatric interview, one of which included only 14 patients treated over 8 weeks and the other, 30 patients over 6 months. Neither showed an antidepressant treatment effect. In one of these trials, there was a higher withdrawal rate in the sertraline group due to adverse events. However, all participants in the present study underwent a structured psychiatric evaluation to rule in MDD and received an adequate dose and duration of treatment to elicit a clinically meaningful effect.
The retention rate in the current study was much higher than in other antidepressant trials involving patients with CKD, comparable with large trials among participants without CKD. There was a high percentage (92%) of patients who completed 6 weeks and 84% who completed 12 weeks (the entire trial duration). In addition, the run-in period successfully minimized nonadherence.
A high placebo response has been postulated to be associated with negative trials of antidepressant medications. However, the 4-point decrease in the QIDS-C16 score in the placebo group is lower than what is considered a “high” placebo response rate observed in depression treatment trials. A meta-analysis of 169 randomized placebo-controlled trials of antidepressant monotherapy concluded that a placebo response rate of less than 30% provided the best chance of finding efficacy in drug-placebo comparisons. Thus, a “high” placebo response rate is an unlikely explanation for a lack of sertraline efficacy in this study. If sertraline did have any effect, it was significantly smaller than postulated in the sample size calculations and clinically irrelevant. In addition, use of a measurement-based care design to bring each participant to the highest individually tolerable dose resulted in adequate dosing (median, 150 mg/d) compared with lower achieved SSRI doses in other large trials among patients with chronic diseases that did not show SSRI treatment efficacy compared with placebo. The mean (SD) sertraline doses were 65 (29) mg/d and 68.8 (40.1) mg/d in trials of patients with congestive heart failure and ischemic heart disease, respectively, and the mean (SD) escitalopram dose was 15.8 (6.4) mg/d in a more recent large congestive heart failure trial. Therefore, inadequate dosing would not explain the lack of effect, and it is unlikely that sertraline is effective for depression treatment in this patient population.
Another reason for lack of efficacy could be that depression comorbid with chronic disease is a different clinical entity than psychiatric MDD among those without comorbidity and depression that is not responsive to SSRI medications. The current study included participants with medical multimorbidity in addition to non–dialysis-dependent CKD (58% with diabetes, 27% with metabolic syndrome, and 32% with congestive heart failure), which makes it challenging to differentiate whether lack of response to sertraline was due to the presence of CKD or other chronic illnesses. These findings are in line with increasing evidence from well-powered trials among patients with other chronic medical conditions, such as asthma, ischemic heart disease, and congestive heart failure, that found SSRIs were no more efficacious than placebo for treating depression. Another potential reason for lack of efficacy is that 84% of participants were unemployed, and socioeconomic factors such as unemployment were shown to be associated with a low likelihood of antidepressant treatment response.
In addition to investigating the efficacy of a commonly prescribed SSRI among patients with CKD, this study assessed adverse events and tolerability. Few data exist regarding SSRI safety among patients with CKD because such patients have been excluded from large antidepressant medication trials. Patients with advanced CKD are at a higher risk for antidepressant adverse events due to the potential accumulation of toxic metabolite effects in the setting of reduced glomerular filtration rate; increased risk of drug-drug interactions; cardiac sequelae (ie, QTc-interval prolongation and orthostatic hypotension); and increased bleeding from SSRI-induced decreased platelet aggregability, which is particularly problematic among patients with advanced CKD.
Sertraline was chosen because it is primarily metabolized by the liver. Its active metabolite, N-desmethylsertraline, is further metabolized to an inactive form before renal excretion. In addition, the Sertraline Antidepressant Heart Attack Randomized Trial found sertraline to be safe among patients with cardiovascular disease. Although sertraline did not result in increased serious adverse events or bleeding, it did increase the rate of adverse gastrointestinal symptoms. These types of adverse events are particularly undesirable among patients with advanced CKD because they are already prone to uremic symptoms such as nausea and vomiting.
Limitations
This study has several limitations. Although the duration was longer than previous studies, it did not assess the long-term effects of antidepressant treatment in CKD. The duration was designed to minimize potential adverse events while allowing adequate drug exposure for a meaningful response. The largest trial of MDD treatment in the general population also assessed efficacy over 12 weeks and showed that a substantial portion of patients who achieved response or remission did so after 8 weeks of treatment with an SSRI. In addition, trials with longer durations (6 and 18 months) among patients with other chronic diseases did not reveal efficacy of SSRI vs placebo. The potential benefit of SSRIs for the treatment of more severe depression may also deserve exploration. Although patients with dialysis-dependent CKD were not included, there is a substantially larger population of patients with non–dialysis-dependent CKD, and the majority of patients with CKD die of cardiovascular disease before reaching end-stage kidney disease. Identifying interventions that could affect outcomes earlier during the disease duration is imperative.
The efficacy and safety of interventions for the treatment of depression among dialysis-dependent patients, a population in which the burden of symptoms, demands of treatment, and propensity for adverse drug reactions are substantially higher than among patients with non–dialysis-dependent CKD, deserve further investigation. Although the current study involved a single geographic area, the demographic breakdown was racially and ethnically diverse, regionally representative, and consistent with published data from other large nationally representative CKD cohorts. The current study did include a larger proportion of black participants than previously reported in some CKD cohorts. However, given that the ratio of adjusted incidence rates for CKD in 2014 for blacks vs whites was 3.1, inclusion of a large proportion of blacks is clinically relevant. Twenty-two percent of study participants had a history of drug abuse; however, it is uncertain whether this factor is generalizable. The inclusion of non-Veteran patients from the University of Texas Southwestern Medical Center and Parkland Hospital increases the generalizability to females with CKD. Future studies need to explore whether non-SSRI medications or nonpharmacological therapies can result in improvement in depressive symptoms among patients with non–dialysis-dependent CKD, who are at a disproportionately higher risk for depression and its complications than the general population.
Conclusions
Among patients with non–dialysis-dependent CKD and MDD, treatment with sertraline compared with placebo for 12 weeks did not significantly improve depressive symptoms. These findings do not support the use of sertraline to treat MDD in patients with non–dialysis-dependent CKD.
Trial protocol
Data analysis plan
eFigure. Randomized double-blinded placebo-controlled trial of sertraline treatment of depression in patients with chronic kidney disease
eTable. Treatment efficacy endpoints at exit
References
- 1.United States Renal Data System 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016. [Google Scholar]
- 2.Hedayati SS, Minhajuddin AT, Toto RD, et al. . Prevalence of major depressive episode in CKD. Am J Kidney Dis. 2009;54(3):424-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer S, Vecchio M, Craig JC, et al. . Prevalence of depression in chronic kidney disease. Kidney Int. 2013;84(1):179-191. [DOI] [PubMed] [Google Scholar]
- 4.Hedayati SS, Minhajuddin AT, Afshar M, et al. . Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303(19):1946-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedayati SS, Bosworth HB, Briley LP, et al. . Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int. 2008;74(7):930-936. [DOI] [PubMed] [Google Scholar]
- 6.Hedayati SS, Grambow SC, Szczech LA, et al. . Physician-diagnosed depression as a correlate of hospitalizations in patients receiving long-term hemodialysis. Am J Kidney Dis. 2005;46(4):642-649. [DOI] [PubMed] [Google Scholar]
- 7.Kimmel PL, Peterson RA, Weihs KL, et al. . Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int. 2000;57(5):2093-2098. [DOI] [PubMed] [Google Scholar]
- 8.Palmer SC, Vecchio M, Craig JC, et al. . Association between depression and death in people with CKD. Am J Kidney Dis. 2013;62(3):493-505. [DOI] [PubMed] [Google Scholar]
- 9.Boulware LE, Liu Y, Fink NE, et al. . Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease. Clin J Am Soc Nephrol. 2006;1(3):496-504. [DOI] [PubMed] [Google Scholar]
- 10.Weisbord SD, Fried LF, Arnold RM, et al. . Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16(8):2487-2494. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal Asher D, Ver Halen N, Cukor D. Depression and nonadherence predict mortality in hemodialysis treated end-stage renal disease patients. Hemodial Int. 2012;16(3):387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glassman AH, O’Connor CM, Califf RM, et al. . Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701-709. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi MH, Fava M, Wisniewski SR, et al. . Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252. [DOI] [PubMed] [Google Scholar]
- 14.Angermann CE, Gelbrich G, Störk S, et al. . Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression. JAMA. 2016;315(24):2683-2693. [DOI] [PubMed] [Google Scholar]
- 15.Jain N, Trivedi MH, Rush AJ, et al. . Rationale and design of the Chronic Kidney Disease Antidepressant Sertraline Trial (CAST). Contemp Clin Trials. 2013;34(1):136-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedayati SS, Minhajuddin AT, Toto RD, et al. . Validation of depression screening scales in patients with CKD. Am J Kidney Dis. 2009;54(3):433-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain N, Carmody T, Minhajuddin AT, et al. . Prognostic utility of a self-reported depression questionnaire versus clinician-based assessment on renal outcomes. Am J Nephrol. 2016;44(3):234-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheehan DV, Lecrubier Y, Sheehan KH, et al. . The Mini-International Neuropsychiatric Interview (MINI). J Clin Psychiatry. 1998;59(suppl 20):22-33. [PubMed] [Google Scholar]
- 19.Trivedi MH, Rush AJ, Wisniewski SR, et al. . Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D. Am J Psychiatry. 2006;163(1):28-40. [DOI] [PubMed] [Google Scholar]
- 20.Rush AJ, Trivedi MH, Ibrahim HM, et al. . The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR). Biol Psychiatry. 2003;54(5):573-583. [DOI] [PubMed] [Google Scholar]
- 21.Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale. Br J Psychiatry. 2002;180:461-464. [DOI] [PubMed] [Google Scholar]
- 22.Hays RD, Kallich JD, Mapes DL, et al. . Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3(5):329-338. [DOI] [PubMed] [Google Scholar]
- 23.Levine J, Schooler NR. SAFTEE. Psychopharmacol Bull. 1986;22(2):343-381. [PubMed] [Google Scholar]
- 24.Wisniewski SR, Rush AJ, Balasubramani GK, et al. . Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006;12(2):71-79. [DOI] [PubMed] [Google Scholar]
- 25.Kirsch I, Deacon BJ, Huedo-Medina TB, et al. . Initial severity and antidepressant benefits. PLoS Med. 2008;5(2):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran D, Molenberghs G, Thijs H, Verbeke G. Sensitivity analysis for pattern mixture models. J Biopharm Stat. 2004;14(1):125-143. [DOI] [PubMed] [Google Scholar]
- 27.Friedli K, Guirguis A, Almond M, et al. . Sertraline versus placebo in patients with major depressive disorder undergoing hemodialysis. Clin J Am Soc Nephrol. 2017;12(2):280-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koo JR, Yoon JY, Joo MH, et al. . Treatment of depression and effect of antidepression treatment on nutritional status in chronic hemodialysis patients. Am J Med Sci. 2005;329(1):1-5. [DOI] [PubMed] [Google Scholar]
- 29.Taraz M, Khatami MR, Dashti-Khavidaki S, et al. . Sertraline decreases serum level of interleukin-6 (IL-6) in hemodialysis patients with depression. Int Immunopharmacol. 2013;17(3):917-923. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy SH, Craven JL, Rodin GM. Major depression in renal dialysis patients: an open trial of antidepressant therapy [published correction appears in J Clin Psychiatry. 1989;50(4):148]. J Clin Psychiatry. 1989;50(2):60-63. [PubMed] [Google Scholar]
- 31.Turk S, Atalay H, Altintepe L, et al. . Treatment with antidepressive drugs improved quality of life in chronic hemodialysis patients. Clin Nephrol. 2006;65(2):113-118. [DOI] [PubMed] [Google Scholar]
- 32.Blumenfield M, Levy NB, Spinowitz B, et al. . Fluoxetine in depressed patients on dialysis. Int J Psychiatry Med. 1997;27(1):71-80. [DOI] [PubMed] [Google Scholar]
- 33.Wuerth D, Finkelstein SH, Kliger AS, Finkelstein FO. Chronic peritoneal dialysis patients diagnosed with clinical depression. Semin Dial. 2003;16(6):424-427. [DOI] [PubMed] [Google Scholar]
- 34.Atalay H, Solak Y, Biyik M, et al. . Sertraline treatment is associated with an improvement in depression and health-related quality of life in chronic peritoneal dialysis patients. Int Urol Nephrol. 2010;42(2):527-536. [DOI] [PubMed] [Google Scholar]
- 35.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375(9715):686-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connor CM, Jiang W, Kuchibhatla M, et al. . Safety and efficacy of sertraline for depression in patients with heart failure. J Am Coll Cardiol. 2010;56(9):692-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown ES, Vigil L, Khan DA, et al. . A randomized trial of citalopram versus placebo in outpatients with asthma and major depressive disorder. Biol Psychiatry. 2005;58(11):865-870. [DOI] [PubMed] [Google Scholar]
- 38.Jain FA, Hunter AM, Brooks JO III, Leuchter AF. Predictive socioeconomic and clinical profiles of antidepressant response and remission. Depress Anxiety. 2013;30(7):624-630. [DOI] [PubMed] [Google Scholar]
- 39.Angermann CE, Gelbrich G, Störk S, et al. . Rationale and design of a randomised, controlled, multicenter trial investigating the effects of selective serotonin re-uptake inhibition on morbidity, mortality and mood in depressed heart failure patients (MOOD-HF). Eur J Heart Fail. 2007;9(12):1212-1222. [DOI] [PubMed] [Google Scholar]
- 40.Lash JP, Go AS, Appel LJ, et al. . Chronic Renal Insufficiency Cohort (CRIC) Study. Clin J Am Soc Nephrol. 2009;4(8):1302-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Data analysis plan
eFigure. Randomized double-blinded placebo-controlled trial of sertraline treatment of depression in patients with chronic kidney disease
eTable. Treatment efficacy endpoints at exit