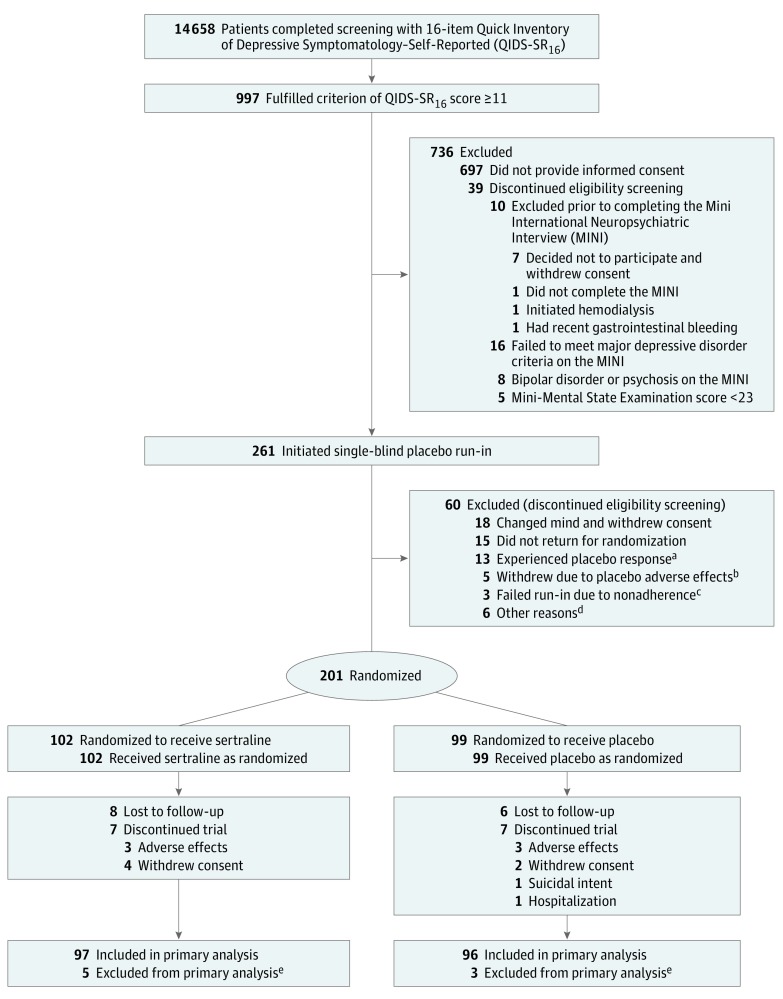
Figure 1. Patient Enrollment in the Chronic Kidney Disease Antidepressant Sertraline Trial (CAST).
aDefined as a 16-item Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C16) score of less than 11 after the placebo run-in phase.
bIncluded self-reported tremor, hypersomnolence, insomnia, diarrhea, and headache.
cDefined as having taken less than 65% of drug by pill count.
dEstimated glomerular filtration rate greater than 60 mL/min/1.73 m2 in 3 patients, ongoing substance dependence in 1 patient, suicidal ideation in 1 patient, and unable to obtain blood for laboratory tests in 1 patient.
eExited the trial prior to the first QIDS-C16 outcomes assessment after randomization (before week 2) and lacked any primary outcome data for analysis. Of the 5 patients in the sertraline group, 1 withdrew consent, 2 withdrew due to adverse effects, and 2 did not return for visits; of the 3 patients in the placebo group, 2 withdrew consent and 1 was hospitalized and no longer wanted to participate.