Key Points
Question
What are choroidal vascular changes seen on optical coherence tomography angiography in acute macular neuroretinopathy?
Findings
In a case series evaluating serial multimodal imaging in 9 eyes of 7 patients with acute macular neuroretinopathy, all had inner choroidal flow void on optical coherence tomography angiography that topographically corresponded to regions of abnormal hyperreflectance of the outer retinal layers on spectral-domain structural optical coherence tomography.
Meaning
These findings suggest areas of inner choroidal vascular flow void on optical coherence tomography angiography are consistently seen in patients with acute macular neuroretinopathy; if confirmed, vascular compromise of the inner choroid may be involved in the pathogenesis of acute macular neuroretinopathy.
Abstract
Importance
Acute macular neuroretinopathy (AMN) is a rare, idiopathic condition resembling other acute maculopathies such as paracentral acute middle maculopathy. The pathophysiology of AMN is not well understood, and the role of the choroid in the pathogenesis of AMN remains controversial.
Objective
To describe initial and serial multimodal imaging findings in AMN, with attention to choroidal vascular changes.
Design, Setting, and Participants
Retrospective case series at a single institution, tertiary referral center. The case series included 7 patients with clinical diagnosis of AMN.
Main Outcomes and Measures
Multimodal imaging findings, including fundus photography, fluorescein angiography, spectral-domain optical coherence tomography (OCT), en face near-infrared imaging, fundus autofluorescence, optical coherence tomography angiography (OCTA), and automated quantification of the regional structural context of choroidal flow interest between different imaging modalities, using an automatic algorithm.
Results
Nine eyes from 7 patients (5 women and 2 men; mean age, 40.1 years) with a diagnosis of AMN were included. Mean duration of follow-up was 11 weeks (range, 1-25 weeks). All eyes had inner choroidal flow void on OCTA that topographically corresponded to regions of abnormal hyperreflectance of the outer retinal layers on spectral-domain OCT and hyporeflectance on en face near-infrared imaging (dice similarity coefficient, 0.76). For each patient, these areas of choroidal flow void on OCTA persisted during the follow-up period, while the abnormal hyperreflectance of outer plexiform layer and inner nuclear layer on spectral-domain OCT was observed to improve.
Conclusions and Relevance
These findings suggest that areas of inner choroidal vascular flow void on OCTA are seen in patients with AMN. These areas may persist weeks after the onset of symptoms and suggest that vascular compromise of the inner choroid may be involved in the pathogenesis of AMN.
This case series describes initial and serial multimodal imaging findings in acute macular neuroretinopathy with attention to choroidal vascular changes.
Introduction
Acute macular neuroretinopathy (AMN), originally described by Bos and Deutman in 1975,1 is a rare, idiopathic condition characterized by acute-onset, paracentral scotoma with dark-reddish, wedge-shaped fundus lesions, with apices pointing toward the fovea.1 Characteristic spectral-domain optical coherence tomography (SD-OCT) findings have been described, including hyperreflectance of the outer retinal layers in the acute phase, particularly the outer plexiform layer (OPL) and outer nuclear layer (ONL), with thinning of these layers seen in follow-up.2,3,4,5 Acute macular neuroretinopathy is more common in young patients, predominantly women, and has been associated with numerous risk factors including oral contraceptive use, caffeine intake, preeclampsia, and viral illness.2 The condition usually improves spontaneously over weeks to months, with some patients experiencing persistent mildly decreased vision or scotomata.1,2,3
The acute onset of symptoms and association with the known circulatory risk factors has implicated vascular compromise as a potential etiology for AMN.1,2 Localization of the SD-OCT abnormalities to the outer retina, including abnormalities in the OPL, led to speculation that deep retinal capillary plexus (DCP) ischemia may play a role, particularly owing to the lack of visible retinal pigment epithelial (RPE) or choroidal changes on OCT or conventional fluorescein angiography (FA).2,3 However, until the advent of OCTA, neither the DCP nor the choriocapillaris could be reliably visualized in vivo with commercially available retinal imaging modalities.
Optical coherence tomography angiography (OCTA) is an emerging imaging modality that allows for visualization of the individual retinal and choroidal vascular networks, including the DCP and choriocapillaris, which are not well visualized by FA or indocyanine green angiography.6 Optical coherence tomography angiography has demonstrated vascular involvement in several idiopathic maculopathies where such vascular insult had only been postulated but not seen on FA or indocyanine green angiography. For example, several studies using OCTA have demonstrated flow loss in the DCP in paracentral acute middle maculopathy (PAMM),7,8,9 which has been initially described as a variant of the AMN phenotype. Similarly, several studies have demonstrated OCTA choroidal flow loss in acute posterior multifocal placoid pigment epitheliopathy (APMPPE), further implicating choroidal vascular insult in its pathogenesis.10,11 Thanos et al12 provided OCTA evidence that choriocapillaris flow loss may also be seen in AMN. However, their report included only 3 eyes from 2 patients and lacked follow-up data to evaluate these abnormalities over time. In addition, the study of regional associations between choroidal flow and structural change has been qualitative.
Here, we present multimodal imaging findings in 9 eyes of 7 patients with AMN. We describe structural and vascular abnormalities across multimodal imaging modalities and correlate these to lesions seen clinically on initial presentation and serial follow-up. Specifically, we analyze vascular flow within the choriocapillaris as seen on OCTA, quantify the associations with regional structure, and describe the evolution of these changes over time relative to abnormalities seen on other multimodal imaging including SD-OCT. Finally, we correlate these findings with histologic descriptions of choroidal anatomy and propose a pathophysiologic mechanism to explain the multimodal imaging findings in AMN compared with other acute idiopathic maculopathies.
Methods
This is a retrospective, observational case series evaluating clinical and multimodal imaging findings for patients diagnosed with AMN at the University of Iowa from January 2016 to April 2017. Approval was obtained from the human subjects committee of the University of Iowa prior to conduct of the study. No patient consent was required because this was a retrospective medical records review. Research presented herein adhered to the tenets of the Declaration of Helsinki and was conducted in accord with regulation set forth by the Health Insurance Portability and Accountability Act. The diagnostic criteria of AMN for inclusion in this study were (1) a history of acute-onset paracentral or central scotoma, (2) characteristic SD-OCT findings including abnormal hyperreflectivity of the OPL and ONL and disruption of the ellipsoid zone (EZ), and (3) teardrop, wedge, or lobular-shaped areas on en face near infrared reflectance (NIR) imaging.
A complete ophthalmologic examination and history were performed and recorded for all patients. Multimodal imaging, including SD-OCT, fundus autofluorescence, NIR imaging (Spectralis HRA + OCT, Heidelberg Engineering), color fundus photography and FA (Topcon), and OCTA (AngioPlex, Cirrus HD-OCT 5000; Carl-Zeiss Meditec Inc) were performed at the time of presentation for each patient. Optical coherence tomography angiography scan protocols included both 6 × 6–mm and 3 × 3–mm scans centered on the fovea for each eye. Spectral-domain OCT, NIR, and OCTA were also performed at follow-up visits. All SD-OCT images were taken using the registration feature, allowing point-to-point comparison of the retinal imaging findings between visits. The multimodal imaging findings were reviewed and analyzed for each patient on initial examination and follow-up visits.
To quantify local associations between OCTA-derived choroidal flow and abnormalities in NIR and OCT, a novel automatic algorithm was developed. In brief, to obtain the neuroretinal and choroidal slabs, we segmented 11 retinal and choroidal surfaces in the OCTA scan using the Iowa Reference Algorithms.13 The neuroretinal and choroidal vascular flow slabs were generated from the internal limiting membrane to OPL and to the choroid layer, respectively, by maximum intensity projection. The NIR and neuroretinal slab were coregistered using an affine transform, using landmarks detected using scale-invariant feature transform.14 The hypointense lesions on NIR and the low flow lesions in the choroidal slab on OCTA were extracted, followed by erosion and dilation. Dice similarity coefficient between the 2 corresponding regions was calculated for each NIR and OCTA pair.
Results
Nine eyes from 7 patients (5 women and 2 men) meeting our study criteria for AMN were included. The demographic characteristics and clinical examination findings are shown in the Table. The mean age was 40.1 years (range, 19-48 years). All but 1 patient (patient 5) presented within 1 week of symptom onset, and presenting visual acuity ranged from 20/15 to 20/40. Mean duration of follow-up was 11 weeks (range, 1-25 weeks). Each affected eye had characteristic teardrop (isolated, oval-shaped), multilobular (multiple, discrete lobules), or confluent multilobular (confluent lesions with lobular edges) areas of hyporeflectance on NIR, with corresponding flow void in the inner choroid on OCTA that was apparent on both the 6 × 6-mm and 3 × 3-mm scans. No areas of flow loss were noted in the superficial or DCP in any of the affected eyes. Median duration of follow-up was 4 weeks, with final visual acuity of 20/20 or better for each affected eye. Areas of choriocapillaris flow loss seen by OCTA remained visible at final follow-up for all involved eyes. Areas of hyporeflectance on NIR correlated well with areas of choroidal flow void on OCTA, with mean dice similarity coefficient of 0.76 (range, 0.49-0.87) (Table; eFigure 1 in the Supplement). Four representative eyes from 3 patients and retinal imaging are presented here.
Table. Demographic Characteristics and Ocular Findings.
| Patient No./Sex/Race/Ethnicity/Age, y | Risk Factors | BCVA at Initial Visit | Duration of Symptoms Before Initial Visit, d | Fundus Examination | Shape of Hyporeflectance in NIR and Inner Choroidal Flow Loss in OCTA | Dice Coefficient Between NIR and OCTA | BCVA at Final Visit | Duration of Follow-up, wk |
|---|---|---|---|---|---|---|---|---|
| 1/F/white/early 20s | ||||||||
| Left eye | None | 20/30 | 5 | Blunted foveal reflex, reddish perifoveal wedge | Multilobular | 0.79 | 20/20 | 4 |
| 2/F/white/late teens | ||||||||
| Right eye | URI, OCP use |
20/20 | 3 | Reddish perifoveal discoloration | Multilobular | 0.87 | 20/20 | 20 |
| Left eye | URI, OCP use |
20/20 | 3 | Reddish perifoveal discoloration | Multilobular | 0.87 | 20/20 | 20 |
| 3/F/white/late 40s | ||||||||
| Right eye | Hypertension, Pancreatitis |
20/40 | 7 | Reddish perifoveal discoloration | Confluent, multilobular |
0.78 | 20/20 | 4 |
| Left | Hypertension, Pancreatitis |
20/40 | 7 | Reddish perifoveal discoloration | Confluent, multilobular |
0.85 | 20/20 | 4 |
| 4/F/Hispanic/mid-30s | ||||||||
| Left eye | Preeclampsia, chronic kidney disease |
20/20 | 1 | Reddish-brown discoloration | Teardrop | 0.62 | 20/20 | 25 |
| 5/M/white/early 40s | ||||||||
| Left eye | Flu vaccine | 20/20 | 21 | Dark-brown discoloration | Confluent, multilobular |
0.49 | 20/20 | 1 |
| 6/F/white/late 20s | ||||||||
| Left eye | Sinus infection, URI |
20/15 | 7 | Reddish-brown discoloration | Teardrop | 0.87 | 20/15 | 24 |
| 7/M/white/early 20s | ||||||||
| Right eye | History of episcleritis | 20/15 | 7 | Juxtafoveal hypopigmented lesion | Confluent, multilobular |
0.74 | 20/15 | 5 |
Abbreviations: BCVA, best-corrected visual acuity; NIR, near infrared; OCP, oral contraceptive pill; OCTA, optical coherence tomography angiography; URI, upper respiratory tract infection.
Patient 1
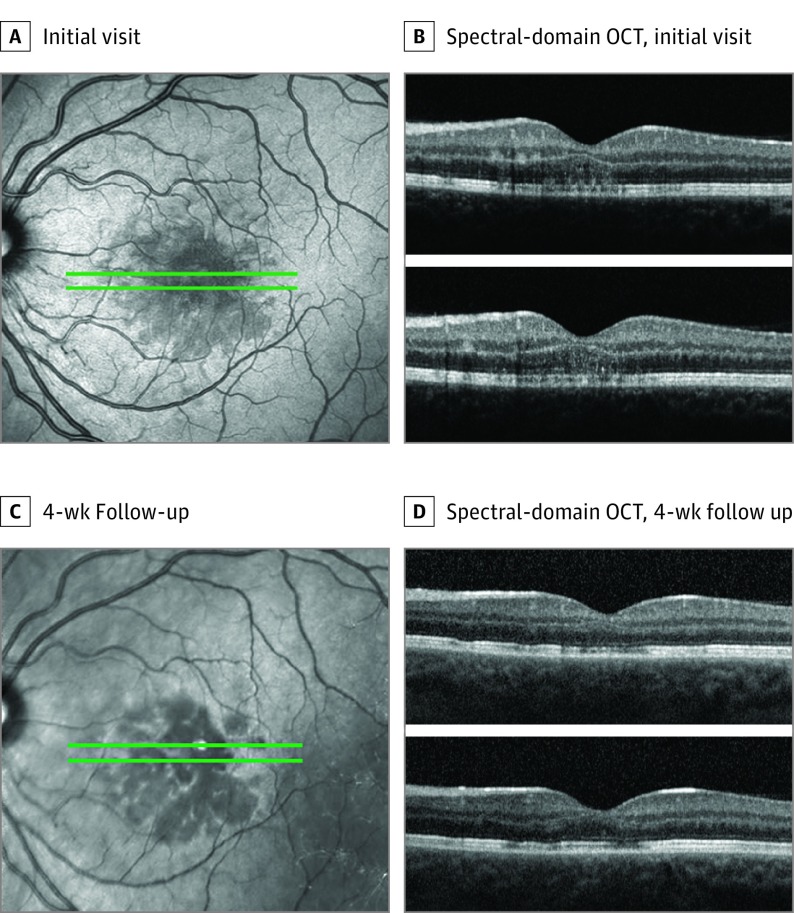
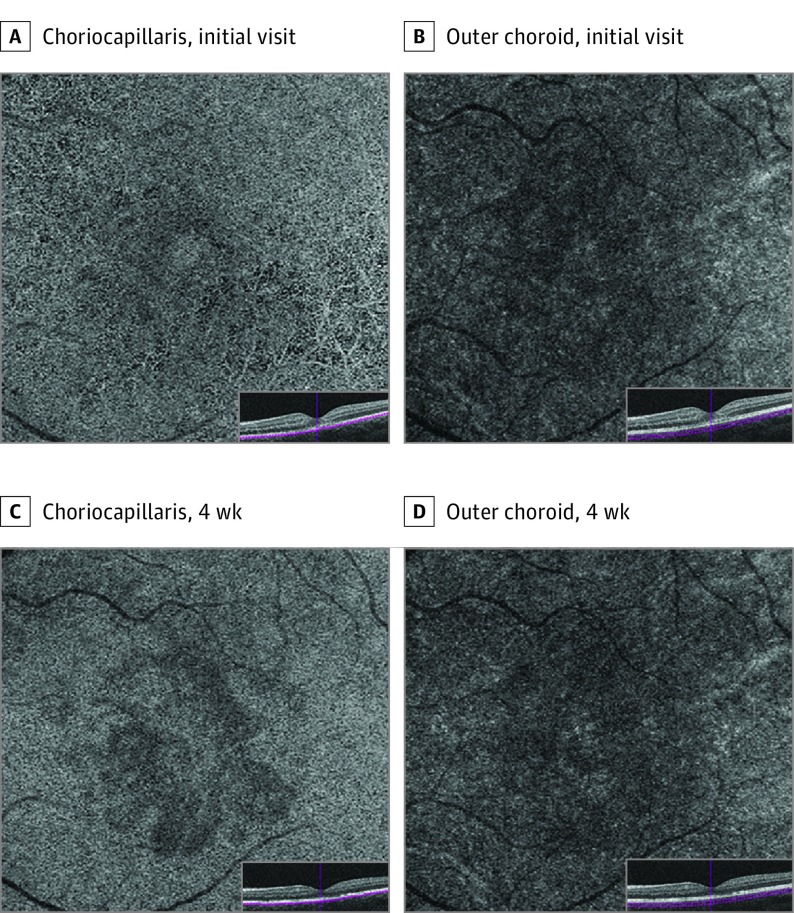
A healthy woman in her early 20s had 5 days of a central scotoma in the left eye. She denied any medication use, recent vaccinations, or illnesses. Best-corrected visual acuity was 20/32 OS. Goldmann visual fields confirmed a central scotoma in the left eye. Anterior segment examination was unremarkable. Dilated fundus examination showed a perifoveal dark-reddish lesion in the left eye. Fluorescein angiography did not show any filling defects or areas of abnormal fluorescence. Near-infrared imaging showed multilobular, hyporeflective areas that appeared larger than but corresponded to the visible lesion on fundus examination. Spectral-domain OCT demonstrated stippled irregular hyperreflectivities of OPL and ONL and attenuation of EZ. The RPE layer appeared unaffected. The OCTA demonstrated flow void at the level of the choriocapillaris that colocalized with the lesions on NIR and SD-OCT. The superficial retinal capillary plexus, DCP, and outer choroid showed a qualitatively normal flow pattern. After 1 month, the previously noted hyperreflective areas on SD-OCT were less prominent, with thickening of the OPL, thinning of the ONL, and improved continuity of the EZ. En face NIR imaging showed consolidation of the multilobular, hyporeflective areas. The OCTA demonstrated persistent choriocapillaris flow void, which was more readily apparent than on previous images (Figures 1 and 2; eFigure 2 in the Supplement).
Figure 1. Patient 1, Left Eye.
Near infrared images (A and C). Spectral-domain optical coherence tomography (OCT) line scans (B and D).
Figure 2. Patient 1, Left Eye.
Optical coherence tomography (OCT) angiography of choriocapillaris at initial visit (A) and 4-week follow-up (C). Optical coherence tomography angiography of outer choroid at initial visit (A) and 4-week follow-up (C).
Patient 2
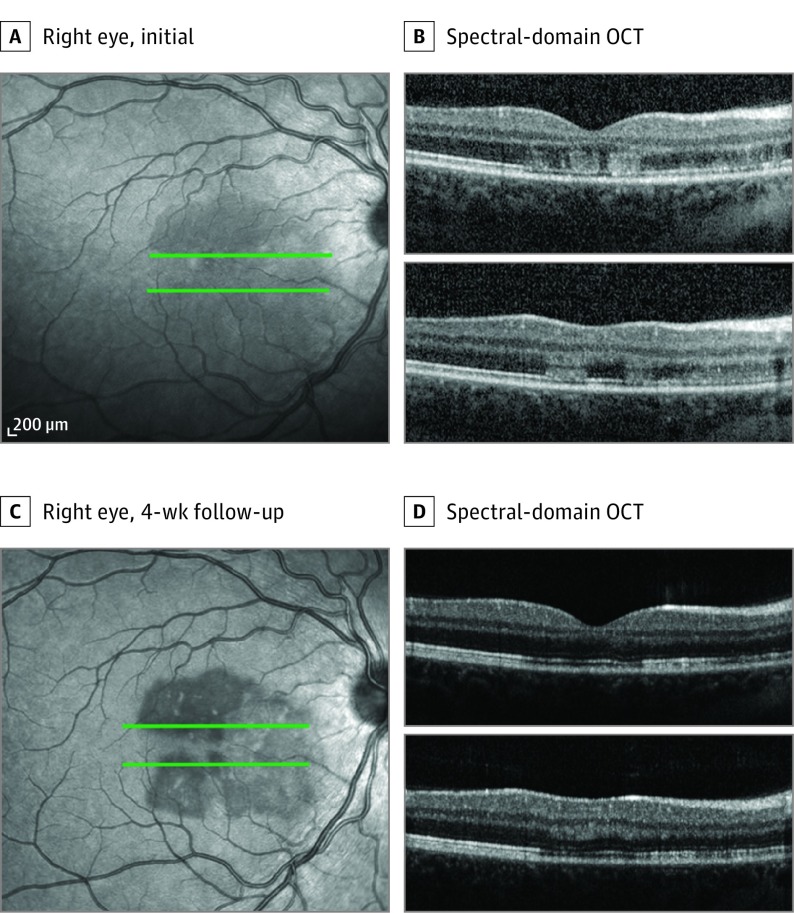
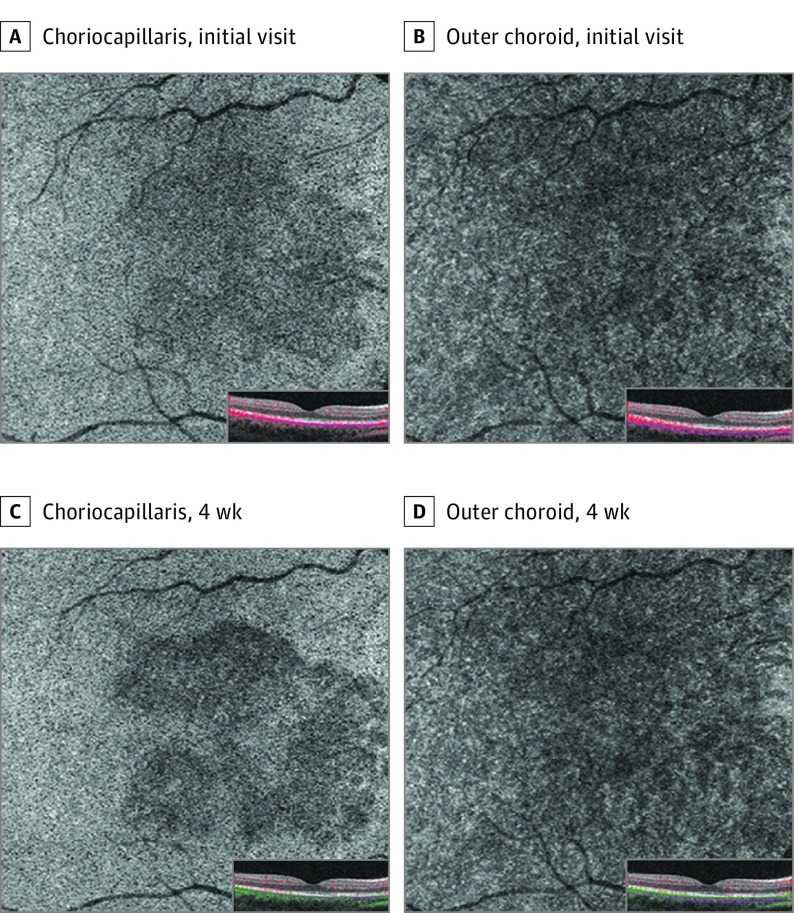
A woman in her late teens receiving daily oral contraceptives had 3 days of bilateral “splotchy” vision. She had an upper respiratory infection about a week prior. Best-corrected visual acuity measured 20/20 OU. Examination showed subtle perifoveal reddish lesions in both eyes. En face NIR imaging showed multilobular, hyporeflective lesions pointing toward the fovea. Spectral-domain OCT demonstrated abnormal segmental hyperreflectivities of the OPL and ONL and disruption of the EZ, with preservation of the RPE. The OCTA demonstrated choriocapillaris flow loss that colocalized with the lesions seen on NIR and SD-OCT. The superficial capillary plexus, DCP, and outer choroid showed no vascular abnormalities. After 1 month, the lesions appeared to have more discrete borders on NIR imaging. Spectral-domain OCT showed near-resolved hyperreflectivity within the outer retinal layers. The OCTA revealed persistent choriocapillaris flow deficits but no visible abnormalities within the other retinal and choroidal vascular layers (Figures 3 and 4; eFigure 3 in the Supplement).
Figure 3. Patient 2, Right Eye .
Near infrared image at presentation (A) and 4 weeks (C) of the right eye. Spectral-domain optical coherence tomography line scans (B and D).
Figure 4. Patient 2, Right Eye.
Optical coherence tomography angiography of choriocapillaris at initial visit (A) and 4-week follow-up (C). Optical coherence tomography angiography of outer choroid at initial visit (A) and 4-week follow-up (C).
Patient 6
A woman in her late 20s had 5 days of decreased central vision in her left eye. When this developed, she was receiving oral amoxicillin for sinusitis. Best-corrected visual acuity measured 20/15 OS, with central scotoma. Dilated fundus examination showed a juxtafoveal teardrop-shaped reddish discoloration. Near-infrared imaging showed a small teardrop-shaped hyporeflective lesion pointing to fovea. Spectral-domain OCT showed a small area of subfoveal hyperreflectance affecting the OPL and ONL with disrupted EZ, while the RPE layer appeared intact. Optical coherence tomography angiography identified choriocapillaris flow void that colocalized with the lesion seen on NIR and SD-OCT. Over the course of 6 months, the outer retinal hyperreflectance on SD-OCT resolved, with residual irregularity of the subfoveal EZ. The teardrop-shaped area of hyporeflectivity on NIR became smaller and less apparent. The area of choriocapillaris flow loss on OCTA persisted (eFigure 4 in the Supplement).
Discussion
Although an underlying vascular etiology for AMN has long been suspected, evidence of the site of compromise, whether within the deep retinal capillary plexus or choriocapillaris, has remained elusive. In our cohort, OCTA revealed choriocapillaris flow void that colocalized to the lesions seen clinically and on multimodal imaging in all affected eyes. Furthermore, dice similarity coefficient between abnormal regions in NIR and choroidal flow void in OCTA, where a novel automatic algorithm allowing a comparison between the areas of interest of 2 different imaging modalities using vascular registration was used, confirmed these findings quantitatively (mean DSC, 0.76, range, 0.49-0.87). This confirms the findings reported by Thanos et al,12 who noted choriocapillaris flow void in 3 eyes of 2 patients. Since that initial report, there has been debate as to whether the flow void signal seen in the choriocapillaris on OCTA represents reduced or halted flow or rather represents an artifact such as from blockage of the flow signal from hyperreflectance of the overlying outer retinal layers.15 The persistence of the OCTA choriocapillaris defects shown in our series provides further evidence that the choriocapillaris flow void found by OCTA represents true flow retardation rather than artifact. In all cases except patient 5, who did not have initial hyperreflective change but had only attenuated EZ in OCT, probably owing to its long duration of onset, the transitory, abnormal hyperreflectance within the OPL and ONL on SD-OCT resolved. Meanwhile, the areas of choriocapillaris flow void on OCTA persisted, suggesting that the darker areas seen on OCTA represent true vascular flow void rather than signal blockage from hyperreflectivity within the overlying retinal layers. Patient 6 represents relatively long follow-up (24 weeks) within our series and demonstrates that choriocapillaris flow loss on OCTA may persist even months after AMN onset, well after the abnormal transient hyperreflectivity seen on SD-OCT resolves, followed by attenuation in the outer retinal layers while RPE layers remain intact.
The size and shape of the choriocapillaris flow loss on OCTA in our AMN cases closely match histologic descriptions of known choriocapillaris structural and functional anatomy. The choriocapillaris is a rich vascular bed comprising a honeycomb configuration of lobular units defined by a central, feeding end-arteriole and surrounding draining venules. A single lobule varies in its geometric configuration, having 3 to 6 sides, forming irregular triangular to hexagonal shapes, with a mean size of 620 to 830 μm in diameter.16,17,18,19 In our case series, we observed characteristic teardrop, multilobular, or confluent multilobular lesions both on NIR imaging and OCTA that demonstrate edge contours with similar footprint size to histologic descriptions of posterior choriocapillaris lobules. Specifically, the teardrop pattern seen on NIR and OCTA imaging is similar to a single choriocapillaris lobule in size and shape. The tendency for these lesions to have apices pointing toward the fovea can be explained by the distribution of the temporal short posterior ciliary artery feeding central choroidal lobules, which have arteries generally located on the foveal side.20 Larger, multilobular lesions suggest involvement of multiple, adjacent juxtafoveal choroidal lobules, including central placoid lesions that appear more confluent and seem to involve the fovea.
Acute macular neuroretinopathy has been associated with numerous vascular risk factors, such as oral contraceptive use, caffeine, epinephrine, and preeclampsia,2 and we propose that mechanisms that compromise the choriocapillaris vasculature may result in decreased choriocapillaris lobular flow seen on OCTA. As a high-flow and low-pressure vascular bed, the choriocapillaris may be susceptible to transient ischemic insult owing to its relative lack of autoregulation in comparison with the retinal blood vessels, vulnerability to slow vascular flow states or steal phenomena, and the presence of α-adrenergic receptors that may make it more susceptible to sympathetic stimuli.21 In AMN, it is possible that vascular spasm, thrombosis, or inflammation at the level of the collecting veins compromises blood flow to single or multiple adjacent lobules owing to increased back flow pressure. Insult to the collecting veins rather than of the central arteriole seems more likely based on 2 main arguments. First, prior studies have shown that within the choriocapillaris, blood does not flow from 1 lobule to the other, meaning that the blood in 1 arteriole is not shared across adjacent segments, whereas blood from adjacent lobules flows to common venules.16 Therefore, to compromise multiple choriocapillaris lobules and create a multilobular or confluent multilobular lesion, either multiple separate sites on adjacent end-arterioles are individually affected, or 1 or more adjacent collecting venules are jointly affected. Secondarily, arteriolar insult would likely lead to more complete choroidal flow loss that would be seen on conventional FA, while venular insult would lead to mildly decreased flow that may not be detected on FA but can be detected by OCTA.
Fawzi et al3,15 have described SD-OCT features of AMN and noted that the initial hyperreflective changes affect the OPL primarily, followed by the ONL and attenuation of the EZ.3,15 Because of the early OPL involvement, the DCP has been suggested as the primary site of insult in AMN cases. However, in our study, none of the 9 eyes with AMN had visible flow abnormalities within the DCP by OCTA. This is consistent with the initial report by Thanos et al,12 who also did not see DCP flow loss in any of the 3 eyes included.12 We hypothesize that the decreased flow in choriocapillaris is the primary insult in AMN, followed by hypoxic insult to the middle, then outer retina, resulting in early hyperreflectivity and eventual atrophic thinning on SD-OCT in these affected areas. The RPE layer may be spared owing to its proximity to the blood flow of the fenestrated inner choroid, whereas the photoreceptors may be preferentially affected owing to their intrinsically high oxygen demand and relative distance from the choriocapillaris.
Sarraf et al9 have defined cases limited to the middle retina as paracentral acute middle maculopathy (PAMM), initially classifying it as AMN variant.9 Numerous studies7,8,22 have since used OCTA in PAMM to demonstrate vascular flow loss in the DCP that correlate to areas of retinal whitening on clinical examination or hyperreflectivity on SD-OCT, suggesting that PAMM is associated with vascular insult to the DCP.7,8,22 The cases included in our study, as well as those by Thanos et al,12 represent classic AMN according to the original description, with SD-OCT changes deep to the OPL, and in none of the 12 eyes from both studies combined was flow loss within the DCP seen. These findings suggest that AMN differs pathophysiologically from PAMM in that AMN results from choroidal vascular insult, whereas PAMM results from DCP insult.
In fact, the etiology of AMN may have more similarities to APMPPE than PAMM, owing to likely and demonstrated involvement of the choroidal vasculature in both conditions. Although APMPPE was considered a retinal pigment epitheliopathy by Gass,23 evidence via OCTA has suggested that choroidal perfusion abnormalities may play a role in the pathophysiology of the condition,10,11,24,25,26 leading some authors to suggest that the name of the condition be changed to acute multifocal placoid choroidopathy.27 Acute macular neuroretinopathy differs from APMPPE in that the latter tends to have prominent FA findings of early choroidal hypofluorescence followed by late hyperfluorescence of distinct, posterior placoid lesions, as well as RPE involvement clinically and on SD-OCT.10,23,28 However, emerging OCTA evidence of choriocapillaris insult in both AMN and APMPPE suggests a possible shared pathophysiologic mechanism and that the 2 conditions may be on the same spectrum of disorders, depending on severity of choroidal involvement.
Limitations
The study is limited by its small sample size, qualitative nature, and variable durations of follow-up. Further longitudinal imaging studies are needed to confirm these findings and provide more insight into the pathogenesis of this rare condition.
Conclusions
To our knowledge, this is the largest case series to date describing OCTA findings in AMN. We demonstrate that OCTA detects choroidal flow abnormalities, which correlate well with the lesions seen clinically on NIR imaging and correspond to histologic descriptions of choroidal anatomy, providing further evidence that vascular insult to the choriocapillaris is involved in the pathogenesis of AMN.
eFigure 1. Automated Quantification of Choroidal Flow Regional Context
eFigure 2. Patient 1, Left Eye.
eFigure 3. Patient 2, Both Eyes.
eFigure 4. Patient 6, Left Eye.
References
- 1.Bos PJ, Deutman AF. Acute macular neuroretinopathy. Am J Ophthalmol. 1975;80(4):573-584. [DOI] [PubMed] [Google Scholar]
- 2.Bhavsar KV, Lin S, Rahimy E, et al. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol. 2016;61(5):538-565. [DOI] [PubMed] [Google Scholar]
- 3.Fawzi AA, Pappuru RR, Sarraf D, et al. Acute macular neuroretinopathy: long-term insights revealed by multimodal imaging. Retina. 2012;32(8):1500-1513. [DOI] [PubMed] [Google Scholar]
- 4.Feigl B, Haas A. Optical coherence tomography (OCT) in acute macular neuroretinopathy. Acta Ophthalmol Scand. 2000;78(6):714-716. [DOI] [PubMed] [Google Scholar]
- 5.Neuhann IM, Inhoffen W, Koerner S, Bartz-Schmidt KU, Gelisken F. Visualization and follow-up of acute macular neuroretinopathy with the Spectralis HRA+OCT device. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):1041-1044. [DOI] [PubMed] [Google Scholar]
- 6.Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(1):45-50. [DOI] [PubMed] [Google Scholar]
- 7.Dansingani KK, Inoue M, Engelbert M, Freund KB. Optical coherence tomographic angiography shows reduced deep capillary flow in paracentral acute middle maculopathy. Eye (Lond). 2015;29(12):1620-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan MA, Rahimy E, Shahlaee A, Hsu J, Ho AC. En face optical coherence tomography imaging of deep capillary plexus abnormalities in paracentral acute middle maculopathy. Ophthalmic Surg Lasers Imaging Retina. 2015;46(9):972-975. [DOI] [PubMed] [Google Scholar]
- 9.Sarraf D, Rahimy E, Fawzi AA, et al. Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013;131(10):1275-1287. [DOI] [PubMed] [Google Scholar]
- 10.Salvatore S, Steeples LR, Ross AH, Bailey C, Lee RW, Carreño E. Multimodal imaging in acute posterior multifocal placoid pigment epitheliopathy demonstrating obstruction of the choriocapillaris. Ophthalmic Surg Lasers Imaging Retina. 2016;47(7):677-681. [DOI] [PubMed] [Google Scholar]
- 11.Park SS, Thinda S, Kim DY, Zawadzki RJ, Werner JS. Phase-variance optical coherence tomographic angiography imaging of choroidal perfusion changes associated with acute posterior multifocal placoid pigment epitheliopathy. JAMA Ophthalmol. 2016;134(8):943-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thanos A, Faia LJ, Yonekawa Y, Randhawa S. Optical coherence tomographic angiography in acute macular neuroretinopathy. JAMA Ophthalmol. 2016;134(11):1310-1314. [DOI] [PubMed] [Google Scholar]
- 13.Garvin MK, Abràmoff MD, Wu X, Russell SR, Burns TL, Sonka M. Automated 3-D intraretinal layer segmentation of macular spectral-domain optical coherence tomography images. IEEE Trans Med Imaging. 2009;28(9):1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowe DG. Object recognition from local scale-invariant features In: The Proceedings of the Seventh IEEE International Conference on 1999. Washington, DC: IEEE Computer Society; 1999: 1150-1157. [Google Scholar]
- 15.Ashraf M, Goldstein D, Fawzi A. Optical coherence tomography angiography: potential artifacts in acute macular neuroretinopathy. JAMA Ophthalmol. 2017;135(6):675-676. [DOI] [PubMed] [Google Scholar]
- 16.Hayreh SS. The choriocapillaris. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1974;192(3):165-179. [DOI] [PubMed] [Google Scholar]
- 17.Torczynski E, Tso MO. The architecture of the choriocapillaris at the posterior pole. Am J Ophthalmol. 1976;81(4):428-440. [DOI] [PubMed] [Google Scholar]
- 18.Yoneya S, Tso MO. Angioarchitecture of the human choroid. Arch Ophthalmol. 1987;105(5):681-687. [DOI] [PubMed] [Google Scholar]
- 19.Yoneya S, Tso MO, Shimizu K. Patterns of the choriocapillaris: a method to study the choroidal vasculature of the enucleated human eye. Int Ophthalmol. 1983;6(2):95-99. [DOI] [PubMed] [Google Scholar]
- 20.Hayreh SS. Submacular choroidal vascular pattern. Experimental fluorescein fundus angiographic studies. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1974;192(3):181-196. [DOI] [PubMed] [Google Scholar]
- 21.Alm A. The effect of sympathetic stimulation on blood flow through the uvea, retina and optic nerve in monkeys (Macacca irus). Exp Eye Res. 1977;25(1):19-24. [DOI] [PubMed] [Google Scholar]
- 22.Sridhar J, Shahlaee A, Rahimy E, et al. Optical coherence tomography angiography and en face optical coherence tomography features of paracentral acute middle maculopathy. Am J Ophthalmol. 2015;160(6):1259-1268.e2. [DOI] [PubMed] [Google Scholar]
- 23.Gass JD. Acute posterior multifocal placoid pigment epitheliopathy. Arch Ophthalmol. 1968;80(2):177-185. [DOI] [PubMed] [Google Scholar]
- 24.Dolz-Marco R, Sarraf D, Giovinazzo V, Freund KB. Optical coherence tomography angiography shows inner choroidal ischemia in acute posterior multifocal placoid pigment epitheliopathy. Retin Cases Brief Rep. 2017;11(suppl 1):S136-S143. [DOI] [PubMed] [Google Scholar]
- 25.Heiferman MJ, Rahmani S, Jampol LM, et al. Acute posterior multifocal placoid pigment epitheliopathy on optical coherence tomography angiography [published online February 1, 2017]. Retina. doi: 10.1097/IAE.0000000000001487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mrejen S, Sarraf D, Chexal S, Wald K, Freund KB. Choroidal involvement in acute posterior multifocal placoid pigment epitheliopathy. Ophthalmic Surg Lasers Imaging Retina. 2016;47(1):20-26. [DOI] [PubMed] [Google Scholar]
- 27.Zhang AY, Han IC, Goldberg MF. Renaming of acute posterior multifocal placoid pigment epitheliopathy (APMPPE) to acute multifocal placoid choroidopathy (AMP-C). JAMA Ophthalmol. 2017;135(3):185. [DOI] [PubMed] [Google Scholar]
- 28.Scarinci F, Fawzi AA, Shaarawy A, Simonett JM, Jampol LM. Longitudinal quantitative evaluation of outer retinal lesions in acute posterior multifocal placoid pigment epitheliopathy using optical coherence tomography. Retina. 2017;37(5):851-857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Automated Quantification of Choroidal Flow Regional Context
eFigure 2. Patient 1, Left Eye.
eFigure 3. Patient 2, Both Eyes.
eFigure 4. Patient 6, Left Eye.