Key Points
Question
Are there any differences in treatment outcomes between combination therapy with intravitreal ranibizumab and verteporfin photodynamic therapy compared with ranibizumab monotherapy in polypoidal choroidal vasculopathy?
Findings
In the multicenter EVEREST II randomized clinical trial, compared with ranibizumab monotherapy, treatment of polypoidal choroidal vasculopathy with ranibizumab plus verteporfin photodynamic therapy resulted in greater visual acuity improvement (8.3 vs 5.1 letters) than monotherapy and complete resolution of lesions with fewer ranibizumab injections.
Meaning
These data suggest ranibizumab plus verteporfin photodynamic therapy should be considered for treatment of eyes with polypoidal choroidal vasculopathy.
Abstract
Importance
Polypoidal choroidal vasculopathy (PCV) is a common subtype of exudative age-related macular degeneration among Asian individuals. To our knowledge, there are no large randomized clinical trials to evaluate intravitreal ranibizumab, with and without verteporfin photodynamic therapy (vPDT), for the treatment of PCV.
Objective
To compare the efficacy and safety of combination therapy of ranibizumab and vPDT with ranibizumab monotherapy in PCV.
Design, Setting, and Participants
A double-masked, multicenter randomized clinical trial of 322 Asian participants with symptomatic macular PCV confirmed by the Central Reading Center using indocyanine green angiography was conducted between August 7, 2013, and March 2, 2017.
Interventions
Participants were randomized 1:1 to ranibizumab, 0.5 mg, and vPDT (n = 168; combination therapy group) or ranibizumab, 0.5 mg, and sham PDT (n = 154; monotherapy group). All participants received 3 consecutive monthly ranibizumab injections, followed by a pro re nata regimen. Participants also received vPDT/sham PDT on day 1, followed by a pro re nata regimen based on the presence of active polypoidal lesions.
Main Outcomes and Measures
Step 1 assessed whether combination therapy was noninferior (5-letter margin) to monotherapy for change in best-corrected visual acuity from baseline and superior in complete polyp regression. If noninferiority was established, step 2 assessed whether combination therapy was superior to monotherapy measured by best-corrected visual acuity change at month 12.
Results
Baseline demographics of the 322 participants were comparable between the treatment groups. Mean (SD) age of the patients was 68.1 (8.8) years, and overall, 69.9% of the patients were men. At baseline, the overall mean best-corrected visual acuity and mean central subfield thickness were 61.1 letters and 413.3 μm, respectively. At 12 months, mean improvement from baseline was 8.3 letters with combination therapy vs 5.1 letters with monotherapy (mean difference, 3.2 letters; 95% CI, 0.4-6.1), indicating that combination therapy met the predefined criterion for noninferiority as well as being superior to monotherapy (P = .01). Combination therapy was also superior to monotherapy in achieving complete polyp regression at month 12 (69.3% vs 34.7%; P < .001). Over 12 months, the combination therapy group received a median of 4.0 ranibizumab injections compared with 7.0 in the monotherapy group. Vitreous hemorrhage was the only ocular serious adverse event (combination therapy group, 1 [0.6%]; monotherapy group, 3 [2.0%]).
Conclusions and Relevance
After 12 months, combination therapy of ranibizumab plus vPDT was not only noninferior but also superior to ranibizumab monotherapy in best-corrected visual acuity and superior in complete polyp regression while requiring fewer injections. Combination therapy should be considered for eyes with PCV.
Trial Registration
clinicaltrials.gov Identifier: NCT01846273.
This randomized clinical trial compares the efficacy and safety of combination therapy of ranibizumab and verteporfin photodynamic therapy with ranibizumab monotherapy in polypoidal choroidal vasculopathy.
Introduction
Polypoidal choroidal vasculopathy (PCV) is an exudative retinal disease characterized by an abnormal subretinal pigment epithelial network of vessels of choroidal origin, ending in aneurysmal dilatations, which appear as spheroidal polyplike structures. Hemorrhage and exudation from this vascular network can lead to chronic, multiple, recurrent serosanguineous detachments of the retinal pigment epithelium and retina. Untreated, the long-term prognosis of PCV is poor.
The pathogenesis of PCV remains unclear; it was initially considered a distinct abnormality of the inner choroidal vasculature; however, the histopathological evidence suggests that PCV is a variant of type I or occult choroidal neovascularization seen in neovascular age-related macular degeneration (nAMD), located above or within the Bruch membrane. Furthermore, studies within the past decade show that systemic and genetic risk factors for PCV and typical nAMD appear to be fairly similar. Thus, PCV is considered one of the subtypes of nAMD.
Indocyanine green angiography (ICGA) is essential for accurately diagnosing PCV, helping to visualize the hyperfluorescent polypoidal lesions. In general, PCV is reported to be more prevalent in certain racial/ethnic groups, especially in Asian individuals, where the proportion of PCV among nAMD cases varies from 22.3% to 61.6%. However, with increased use of ICGA and advances in other diagnostic techniques, a rise in the frequency of PCV diagnosis has been observed across all patient populations.
The anti–vascular endothelial growth factor agent ranibizumab, with or without verteporfin photodynamic therapy (vPDT), has shown efficacy in improving visual outcomes and diminishing polypoidal lesions in patients with PCV. The EVEREST study was a randomized clinical trial in 61 participants that showed that combination therapy was significantly superior to ranibizumab monotherapy in achieving complete polyp regression over 6 months. Although best-corrected visual acuity (BCVA) also improved in participants treated with either combination therapy or ranibizumab monotherapy, the study was not powered to compare the effects of these treatment modalities on BCVA gains and did not evaluate results beyond 6 months. Therefore, we conducted the 24-month EVEREST II trial to compare the long-term effect of combination therapy vs ranibizumab monotherapy in a large Asian patient population with symptomatic macular PCV. Here, we report the 12-month primary and secondary outcomes.
Methods
Study Design
The EVEREST II trial was a 24-month multicenter, randomized, double-masked study designed to compare the efficacy and safety profile of ranibizumab, 0.5 mg, and vPDT combination therapy with ranibizumab, 0.5 mg, monotherapy in participants with symptomatic macular PCV from Hong Kong, Japan, South Korea, Malaysia, Singapore, Taiwan, and Thailand. The study was conducted in accordance with the Declaration of Helsinki and Tripartite International Council on Harmonization Good Clinical Practice Guidelines and applicable local regulations. The study protocol was reviewed and approved by an independent ethics committee or institutional review board at each center. All participants provided written informed consent. The trial protocol and statistical analysis plan are available in Supplement 1.
Participants
The study population consisted of treatment-naive participants 18 years and older with symptomatic macular PCV, as defined by the presence of active macular polypoidal lesions on ICGA and by the presence of serosanguineous maculopathy on color fundus photography and fluorescein angiography. The presence of PCV in 1 study eye and eligibility for enrollment were confirmed by the Central Reading Center (Fundus Image Reading Centre, Singapore) using a standardized reading protocol using well-defined grading criteria as in EVEREST. The eligible BCVA letter score range was between 78 and 24 (approximately 20/32 to 20/320 Snellen equivalent), measured using Early Treatment Diabetic Retinopathy Study visual acuity charts at 4 m following refraction.
Details of the patient inclusion and exclusion criteria are listed in the eMethods in Supplement 2.
Randomization and Treatment
Participants, evaluating investigators, vision examiners, and Central Reading Center graders were masked to the treatment. Separate unmasked investigators (treating physicians) performed the treatments. All eligible participants were randomized 1:1 to either combination therapy with ranibizumab, 0.5 mg, and standard fluence vPDT or ranibizumab, 0.5 mg, monotherapy (with sham PDT). Randomization was balanced by site (eMethods in Supplement 2).
All participants were assessed monthly. An intravitreal ranibizumab injection (0.5 mg/0.05 mL) was administered on day 1 (baseline) and at months 1 and 2, followed by a pro re nata (PRN) regimen according to the protocol-specific retreatment criteria, with at least a 28-day interval between 2 ranibizumab treatments (eMethods and eFigure 1 in Supplement 2). On day 1, participants in the combination group were infused with intravenous verteporfin (6 mg/m2), and those in the monotherapy group were infused with 5% dextrose solution. Fifteen minutes after the start of infusion, laser (light dose, 50 J/cm2; dose rate, 600 mW/cm2; wavelength, 689 nm) was applied onto the whole lesions in the study eye for 83 seconds. Photodynamic therapy tubing was covered with foil or a blanket. Thereafter, vPDT or sham PDT was administered on a PRN basis from month 3 onwards per the protocol-specific retreatment criteria (eMethods and eFigure 1 in Supplement 2), with at least a 3-month interval between 2 vPDT or sham PDT treatments. As per protocol criteria, fellow eyes that developed macular pathologies were appropriately treated (Trial Protocol in Supplement 1).
Study Objectives
The primary objectives were to demonstrate that combination therapy was (1) noninferior to ranibizumab monotherapy in participants with symptomatic macular PCV with respect to change in BCVA (Early Treatment Diabetic Retinopathy Study letters) from baseline to month 12 with a predefined noninferiority margin of 5 letters and (2) superior with respect to complete polyp regression as assessed by ICGA at month 12. Once this was established, the next step was to show the superiority of combination therapy vs ranibizumab monotherapy with respect to BCVA change from baseline to month 12. The secondary objectives included additional functional and anatomical outcomes, treatment exposure, and safety and tolerability for both treatments up to month 12.
Efficacy Assessments
Efficacy assessments included both functional (BCVA) and multimodal image (ICGA, fluorescein angiography, color fundus, and spectral-domain optical coherence tomography) evaluations of the study eye. Disease activity was assessed based on BCVA loss, spectral-domain optical coherence tomography, ICGA, fluorescein angiography, and color fundus anomalies (eMethods in Supplement 2).
Safety Assessments
Adverse events (AEs) were assessed at each visit.
Statistical Analysis
A sample size of 160 participants per treatment group was estimated to appropriately power the prespecified primary analysis, with the combined power to achieve a 1-sided noninferiority margin of 5 letters between combination therapy and ranibizumab monotherapy with respect to the BCVA change from baseline to month 12, superiority with respect to complete polyp regression, and superiority with respect to BCVA change from baseline at the 1-sided level of α = .025 was at least 87.0%.
The primary efficacy objective was tested based on an analysis of covariance model including treatment group as a factor and (centered) baseline BCVA as a continuous variable for testing noninferiority/superiority of BCVA change from baseline and on a Fisher test to evaluate for superiority with respect to complete polyp regression (eMethods in Supplement 2). The multiple 1-sided α-level of .025 was to be maintained by applying a sequentially rejecting multiple testing procedure (steps 1 and 2).
Baseline demographics and disease characteristics are presented using descriptive statistics. The primary analysis was conducted on the full analysis set (FAS) using the last observation carried forward approach for imputation of the missing data. The FAS comprised all participants who were assigned to a treatment regimen. The secondary analyses were conducted on the study eye of participants in the FAS. The safety analysis was descriptive and conducted on the safety set that consisted of all participants who received at least 1 application of study treatment and had at least 1 postbaseline safety assessment.
Results
Patient Disposition and Baseline Characteristics
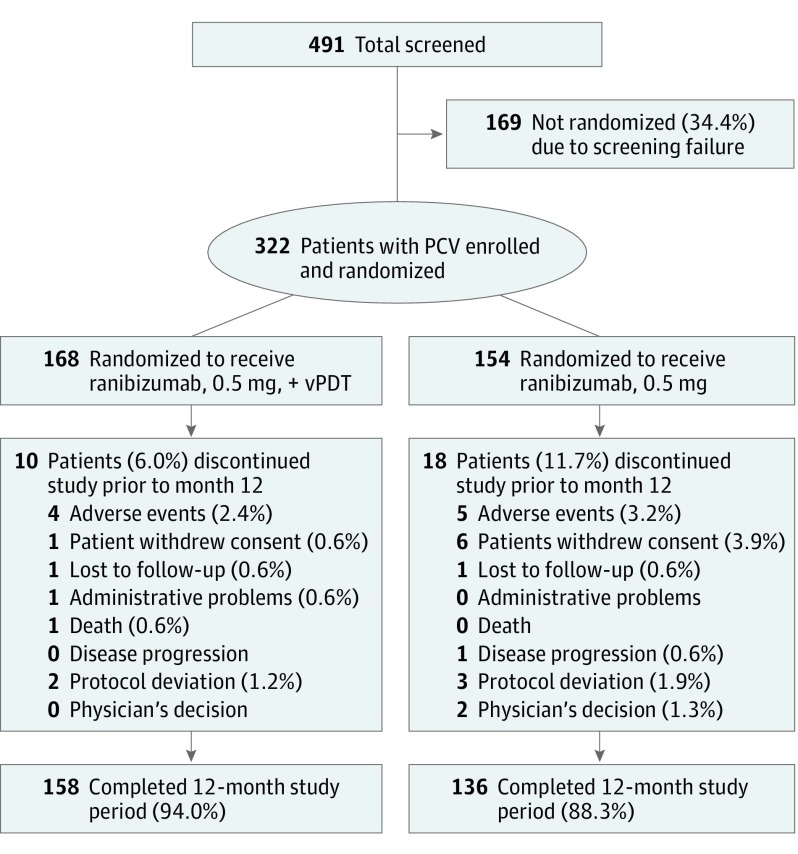
In total, 322 participants were randomized to receive combination therapy (n = 168) or ranibizumab monotherapy (n = 154; Figure 1). Five participants without active polypoidal lesions were randomized in error before Central Reading Center confirmation.
Figure 1. Patient Disposition (Randomized Set).
Randomized set consisted of all randomized participants. Percentages are based on the total number of participants in the randomized set in the respective treatment groups. The 5 participants discontinued from the study owing to protocol deviation were enrolled before the Central Reading Center confirmed polypoidal choroidal vasculopathy (PCV) diagnosis. One of the 2 participants whom the physician decided to withdraw did not respond to treatment and the primary investigator decided to change the treatment. In the other case, there were no documented reasons but the participant did not experience any adverse events. Spectral-domain optical coherence tomography, color fundus photography, fluorescein angiography, and indocyanine green angiography were assessed by the Central Reading Center.
Baseline characteristics were comparable between groups. Overall, the mean (SD) age of participants was 68.1 (8.8) years, and most participants were men (69.9%; Table). The mean baseline BCVA letter score was similar between the combination (61.1 [approximate Snellen equivalent, 20/63]) and monotherapy groups (61.2 [approximate Snellen equivalent, 20/63]; Table). Most study eyes had occult with no classic component lesion types at baseline (Table). Overall, 294 participants completed the first 12 months of the study (158 in the combination arm and 136 in the monotherapy arm; Figure 1).
Table. Patient Demographics, Baseline Disease, and Ocular Characteristics (Randomized Set).
| Parameter | No. (%)a | |
|---|---|---|
| Ranibizumab, 0.5 mg, + vPDT (n = 168) |
Ranibizumab, 0.5 mg (n = 154) |
|
| Age, y | ||
| No. | 168 | 154 |
| Mean (SD) | 68.0 (8.5) | 68.2 (9.0) |
| Age category, y | ||
| <50 | 0 | 4 (2.6) |
| 50-<65 | 57 (33.9) | 53 (34.4) |
| 65-<75 | 73 (43.5) | 56 (36.4) |
| 75-<85 | 33 (19.6) | 34 (22.1) |
| ≥85 | 5 (3.0) | 7 (4.5) |
| Sex | ||
| Male | 109 (64.9) | 116 (75.3) |
| Female | 59 (35.1) | 38 (24.7) |
| Race/ethnicity | ||
| Chinese | 64 (38.1) | 59 (38.3) |
| Indian (Indian subcontinent) | 3 (1.8) | 2 (1.3) |
| Japanese | 46 (27.4) | 38 (24.7) |
| Other | 55 (32.7) | 55 (35.7) |
| BCVA letter score | ||
| No. | 168 | 153 |
| Mean (SD) | 61.1 (12.6) | 61.2 (13.9) |
| Categorized BCVA letter score (approximate Snellen equivalent) | ||
| <39 (Worse than 20/160) | 8 (4.8) | 11 (7.1) |
| 39 -54 (20/160 to Worse than 20/80) | 34 (20.2) | 27 (17.5) |
| ≥54 -<74) (20/80 to Worse than 20/32) | 97 (57.7) | 87 (56.5) |
| ≥74 (20/32 or Better) | 29 (17.3) | 28 (18.2) |
| Missing | 0 | 1 (0.6) |
| Central subfield thickness, µm | ||
| No. | 159 | 149 |
| Mean (SD) | 415.9 (143.7) | 410.4 (170.9) |
| Type of lesion, No. (%) | ||
| 100% classic | 2 (1.2) | 1 (0.6) |
| Predominantly classic | 2 (1.2) | 0 |
| Minimally classic | 9 (5.4) | 16 (10.4) |
| Occult with no classic component | 139 (82.7) | 124 (80.5) |
| Cannot grade | 16 (9.5) | 13 (8.4) |
| Presence of massive submacular hemorrhage | ||
| No | 147 (87.5) | 135 (87.7) |
| Yes | 19 (11.3) | 15 (9.7) |
| Cannot grade | 2 (1.2) | 4 (2.6) |
| Presence of serosanguinous hemorrhage | ||
| No | 72 (42.9) | 61 (39.6) |
| Yes | 94 (56.0) | 88 (57.1) |
| Cannot grade | 2 (1.2) | 5 (3.2) |
| Presence of polypoidal lesions | ||
| No | 2 (1.2) | 3 (1.9) |
| Yes | 166 (98.8) | 151 (98.1) |
| Cannot grade | 0 | 0 |
| No. of polypoidal lesions | ||
| 0 | 0 | 0 |
| 1 | 24 (14.3) | 34 (22.1) |
| 2 | 32 (19.0) | 33 (21.4) |
| 3 | 32 (19.0) | 23 (14.9) |
| 4 | 22 (13.1) | 19 (12.3) |
| ≥5 | 56 (33.3) | 42 (27.3) |
| Missing | 2 (1.2) | 3 (1.9) |
| Polyp size, mm2 | ||
| No. | 166 | 151 |
| Mean (SD) | 0.410 (0.426) | 0.379 (0.331) |
| Presence of BVN | ||
| No | 9 (5.4) | 7 (4.5) |
| Yes | 158 (94.0) | 146 (94.8) |
| Cannot grade | 1 (0.6) | 1 (0.6) |
| BVN size, mm2 | ||
| No. | 158 | 146 |
| Mean (SD) | 3.140 (2.765) | 2.614 (2.231) |
Abbreviations: BCVA, best-corrected visual acuity; BVN, branching vascular network.
Percentages are based on total number of participants in the randomized set in the respective treatment group.
Efficacy
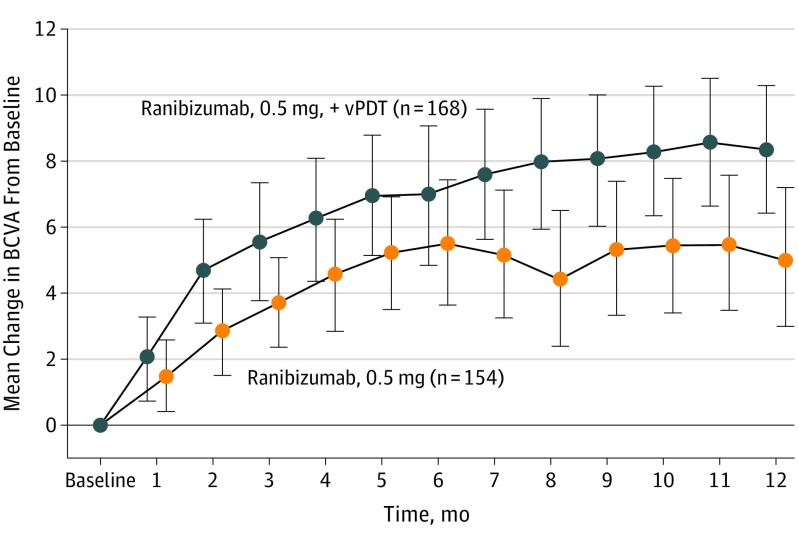
At 12 months, mean improvement from baseline was 8.3 letters with combination therapy vs 5.1 letters with monotherapy (mean difference, 3.2 letters; 95% CI, 0.4-6.1), indicating that combination therapy met the predefined criterion for noninferiority. Combination therapy was statistically superior to ranibizumab monotherapy in improving BCVA from baseline at month 12 (8.3 vs 5.1 letters; P = .01, eTable 1 in Supplement 2). Mean change in BCVA from baseline up to month 12 is shown in Figure 2. Sensitivity analyses using different modeling methods and approaches for handling missing data and outlier values produced similar results (eTables 2-4 in Supplement 2).
Figure 2. Mean Change in Best-Corrected Visual Acuity (BCVA) From Baseline to Month 12 (Full Analysis Set).
The total counts presented are the counts of patients in the specific treatment group who attended the specific visit. These total counts are used as the denominator for the percentages. Error bars represent 95% CIs. vPDT indicates verteporfin photodynamic therapy.
At month 12, 24.5% of participants (n = 38) in the combination arm and 14.0% of participants (n = 19) in the monotherapy arm showed a significant BCVA gain of at least 15 letters (P = .03; eFigure 2 in Supplement 2). At month 12, the proportion of participants with BCVA at least 69 letters of the study eye (approximately 20/40 Snellen equivalent) increased from 32.7% at baseline to 69.0% in the combination arm and from 40.8% at baseline to 58.8% in the monotherapy arm (eFigure 6 in Supplement 2).
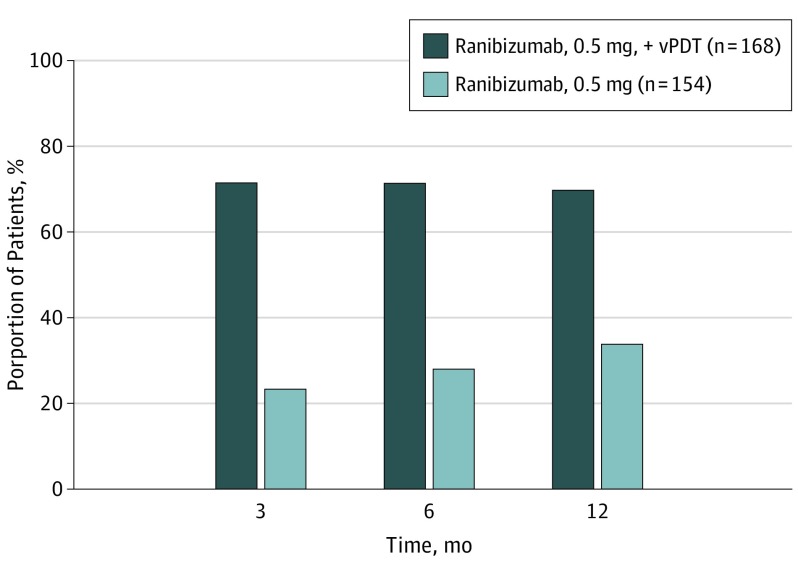
Combination therapy showed statistically significant superiority to ranibizumab monotherapy in achieving complete polyp regression at month 12 as assessed by ICGA (69.3% vs 34.7%; P < .001). The superiority of the combination arm vs the monotherapy arm in achieving complete polyp regression was consistent from months 3 to 12 (Figure 3). In the combination therapy group, 51.6% of participants showed absence of leakage on fluorescein angiography at month 12 vs 25% in the monotherapy group (eFigure 3 in Supplement 2).
Figure 3. Proportion of Participants With Complete Polyp Regression by Study Visits up to Month 12 in Full Analysis Set (FAS).
Assessed by Central Reading Center using indocyanine green angiography. Number values indicate the total number of participants in the FAS in the respective treatment group. Percentages are computed by considering the total number of participants in the respective treatment group who attended the specific visit as a denominator. vPDT indicates verteporfin photodynamic therapy.
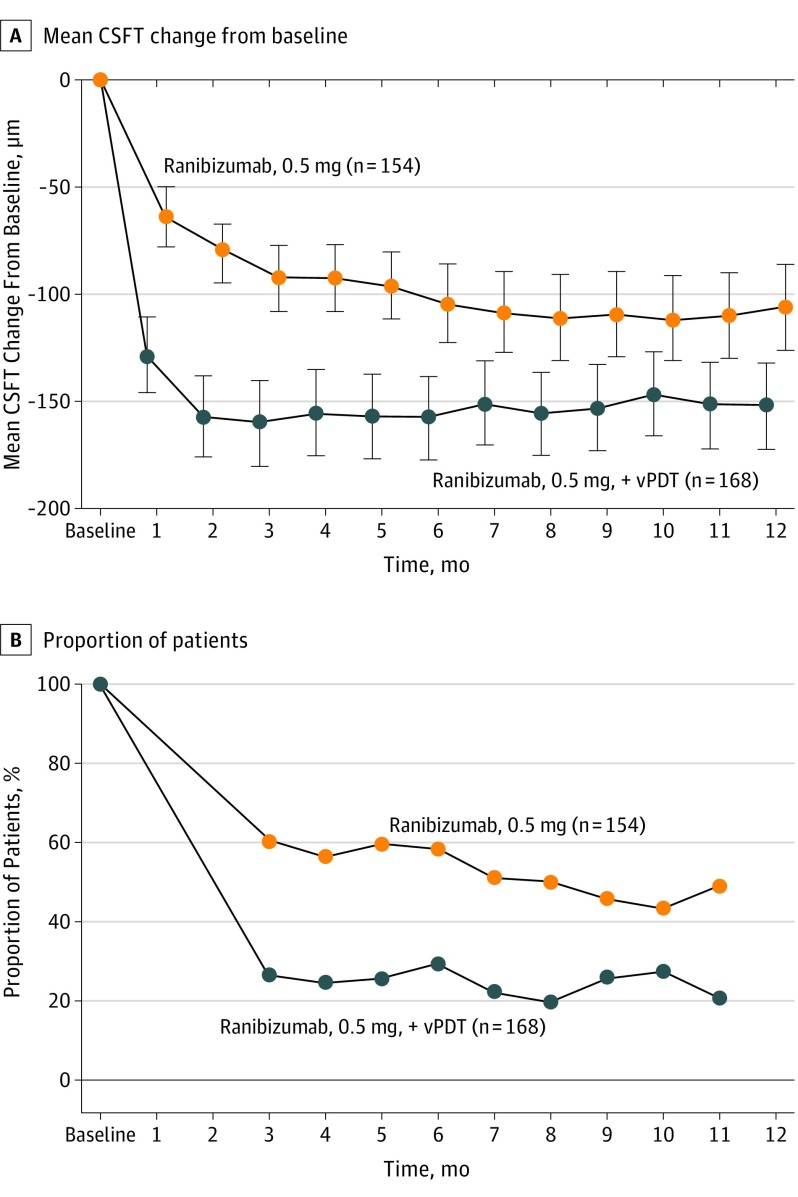
The mean reduction in CSFT from baseline to month 12 was greater in the combination arm than in the monotherapy arm (least squares mean, −164.9 μm vs −113.4 μm, P < .001). Investigator-assessed change in CSFT of the study eye from baseline is illustrated in Figure 4A.
Figure 4. Mean Central Subfield Thickness (CSFT) Change.
Mean CSFT change from baseline to month 12, full analysis set (FAS) (A) and proportion of participants with disease activity by visit (FAS) (B) as assessed by the investigators. Number values indicate the total number of participants in the FAS in the respective treatment group. Percentages are computed by considering the total number of participants in the respective treatment group who attended the specific visit as a denominator. vPDT indicates verteporfin photodynamic therapy.
The proportion of participants with disease activity from month 3 to month 11 was lower in the combination arm than in the monotherapy arm (month 3, 26.4% vs 60.7% and month 11, 20.5% vs 50.0%; Figure 4B). At month 12, serosanguineous maculopathy was present in 14.8% of participants in the combination group (n = 23) and in 8.8% of participants in the monotherapy group (n = 12), whereas submacular hemorrhage (>4 disc areas) was reported in 1.3% of participants in the combination group (n = 2) and 0.7% of participants in the monotherapy group 1). The anatomic outcomes can be clearly seen in an example case provided (eFigure 4 in Supplement 2).
Treatment Exposure
The mean (median) number of ranibizumab injections administered up to month 12 was 5.2 (4) for combination therapy and 7.3 (7) for ranibizumab monotherapy, respectively (eTable 5 in Supplement 2). The difference in the log injection rates between the 2 groups was statistically significant (ratio of injection rates [ranibizumab, 0.5 mg, with vPDT/ranibizumab, 0.5 mg], 0.68; P < .001). Approximately 50.6% of participants (n = 87) in the combination arm required 3 or 4 ranibizumab injections vs 26.2% of participants (n = 39) in the monotherapy arm, while 32.2% of participants (n = 48) in the monotherapy arm required 10 to 12 injections over 12 months compared with 8.7% of participants in the combination arm (n = 15) (eFigure 5A in Supplement 2).
The mean (median) number of vPDT treatments in the combination arm was 1.5 (1), and the mean (median) number of sham PDT treatments in the monotherapy arm was 2.3 (2) (eTable 1 in Supplement 2). Overall, 61.0% of the participants in the combination arm needed only the first vPDT at baseline over the 12 months (eFigure 5B in Supplement 2).
Safety
Vitreous hemorrhage was the only serious ocular AE reported in 1 patient in the combination arm (0.6%) and 3 patients in the monotherapy arm (2.0%) (eTable 6 in Supplement 2). No cases of endophthalmitis or retinal break/detachment were reported in either treatment group. Rates of nonocular serious AEs were comparable between both treatment groups (7.6% in the combination arm vs 7.4% in the monotherapy arm). One patient from the combination group died of chronic obstructive pulmonary disease.
Ocular AEs of the study eye were reported in 26.7% of participants in the combination arm (n = 46) and 25.5% of participants in the monotherapy arm (n = 38) (eTable 7 in Supplement 2). The most common AEs were intraocular pressure increase (5.2% and 4.7%), retinal hemorrhage (3.5% and 0.7%), and conjunctivitis (1.7% and 3.4%) in the combination and monotherapy groups, respectively. Nonocular AEs, regardless of study drug relationship, were reported in 42.4% (n = 73) and 37.6% (n = 56) of participants in the combination and monotherapy groups, respectively.
Discussion
The 12-month results of EVEREST II demonstrated that ranibizumab in combination with vPDT was not only noninferior but also superior to ranibizumab monotherapy in improving vision. In addition, combination therapy was found to be superior to monotherapy in achieving complete polyp regression. Most patients maintained or reached at least 69 letters with both treatment modalities at month 12 (eFigure 6 in Supplement 2). Despite having high baseline BCVA, participants achieved notable BCVA gains of 8.3 letters and 5.1 letters in the combination and monotherapy groups, respectively. Importantly, over 12 months, the median number of ranibizumab injections was 4 in the combination group compared with 7 in the monotherapy group. This difference in intravitreal injections could be significant in terms of cost-effectiveness in many countries.
Our study should be compared with the few prospective PCV trials in the literature. In the 12-month FUJISAN study, participants receiving combination therapy (either at baseline or deferred) had a VA gain of 8.1 and 8.8 letters, respectively. In contrast, participants with PCV in the DRAGON study showed a BCVA gain of 12.7 and 9.4 letters over 12 months with monthly and PRN ranibizumab monotherapy, respectively. The 12-month VA gains in the PLANET study, which assessed fixed dosing of aflibercept in PCV participants with and without rescue PDT, were reported to be 10.8 and 10.7 letters, respectively. Differences in baseline BCVA across the different studies may account for the differences in BCVA gains because poorer baseline BCVA is an important predictor of superior numerical change in BCVA. The baseline BCVA letter score for the ranibizumab PRN monotherapy arm in EVEREST II (61.2 [approximate Snellen equivalent, 20/63]) was higher than the baseline BCVA in the ranibizumab PRN arm in DRAGON (54.6 [approximate Snellen equivalent, 20/80]) and possibly higher than in the aflibercept and sham rescue PDT arm in PLANET (57.7). Importantly, therapeutic outcomes may be underrepresented by simply evaluating VA gains; other factors, such as anatomical responses, polyp regression, and treatment burden, need to be taken into account. In EVEREST, combination therapy was superior to ranibizumab monotherapy in achieving complete polyp regression over 6 months (77.8% vs 28.6%, P = .002). Similarly, in EVEREST II, complete polyp regression rates at months 3, 6, and 12 were consistently higher for combination therapy (71.4%, 71.3%, and 69.7%) vs ranibizumab monotherapy (23.3%, 28.0%, and 33.8%). In FUJISAN, the proportion of participants who showed resolution of polypoidal lesions at month 12 was in broad agreement with EVEREST II whether vPDT was given at baseline or deferred (62.1% vs 54.8%, respectively, P = .53). Importantly, in contrast to these, complete polyp regression rates at month 12 in PLANET were only 38.9% for the aflibercept and sham PDT arm and 44.8% for the aflibercept and rescue PDT arm. This is substantially lower than combination therapy in EVEREST, EVEREST II, or FUJISAN and, in fact, similar to the 12-month complete polyp regression rates in the ranibizumab monotherapy arm in EVEREST II. Taken together, these findings further strengthen the concept that combination therapy achieves superior BCVA outcomes than anti–vascular endothelial growth factor monotherapy along with concomitant higher polyp closure rates. Furthermore, the overall visual and anatomical outcomes of EVEREST II, the largest combination therapy RCT to our knowledge to date, are in concordance with the findings of meta-analyses conducted in 2014 and 2016.
In terms of treatment burden, an increasing concern in the anti–vascular endothelial growth factor therapy era, the mean number of ranibizumab injections required by the combination arm was significantly lower than the monotherapy arm. Over 12 months, 50.6% of the participants in the combination arm required 3 to 4 ranibizumab injections, while only 8.7% of participants in this group required 10 to 12 injections. This reduction in injection number was similar to that observed in other studies evaluating combination therapies for the PCV treatment. In FUJISAN, initial vPDT therapy led to significantly fewer additional ranibizumab treatments after the 3 loading doses vs deferred vPDT therapy. Combination therapy may thus help reduce overall treatment burden and ultimately PCV treatment costs.
In EVEREST II, both treatments showed a considerable 12-month reduction in the proportion of participants with serosanguineous maculopathy and massive submacular hemorrhage, thus allaying physician fears about posttreatment hemorrhage when using vPDT to treat PCV. The safety profiles of both treatment groups were comparable and consistent, with vitreous hemorrhage being the only ocular serious AE reported during 12 months and low rates of retinal hemorrhage in both treatment groups.
Polypoidal choroidal vasculopathy diagnosis has always been challenging owing to its clinical and angiographic resemblance to other retinal pathologies, such as retinal angiomatous proliferation and central serous chorioretinopathy, potentially leading to inappropriate therapy. For example, the efficacy of anti–vascular endothelial growth factor therapy in treating central serous chorioretinopathy is unestablished. Furthermore, in some PCV cases, the polypoidal lesions may be ill-defined or may have extensive bleeding, which renders diagnosis difficult. One of the strengths of EVEREST II was Central Reading Center involvement during screening, using well-defined, stringent criteria modified from EVEREST. This ensured that only definite PCV cases were recruited. The EVEREST criteria have also been validated in real-world settings.
Limitations
The study had a few limitations. The administration of 3 initial monthly injections was presumptive because it was based on nAMD treatment guidelines, which may not apply to combination treatment. Another potential limitation is that only Asian participants with PCV were included, and the results may be ethnospecific. Nevertheless, to our knowledge, no evidence has suggested differential ethnic responses in PCV.
Conclusions
The 12-month EVEREST II results confirm that combination treatment with ranibizumab and vPDT is effective in improving vision of participants with symptomatic macular PCV. Importantly, combination of ranibizumab with vPDT also helps to achieve complete polyp regression, a key clinical outcome for PCV treatment. These functional and anatomical outcomes were achieved with fewer ranibizumab injections over 12 months, thereby reducing treatment burden.
Trial Protocol.
eMethods.
eFigure 1: Retreatment Algorithm
eFigure 2: Categorized Change in BCVA at Month 12 (FAS)
eFigure 3: Proportion of Patients With No Leakage in the Study Eye, by Visit From Months 3 to 12 (FAS)
eFigure 4: Example Case of PCV Patient Treated With Ranibizumab 0.5 mg + vPDT Combination Therapy – Baseline OCT
eFigure 5. Frequency of Ranibizumab Injections Administered Over 12 Months (Safety Set)
eFigure 6: Proportion of Patients With BCVA ≥69 letters at Baseline and Month 12 (FAS)
eTable 1: Treatment Comparison For Change in the Best-Corrected Visual Acuity From Baseline at Month 12 (FAS, LOCF)
eTable 2: Treatment Comparison for Change in the Best-Corrected Visual Acuity From Baseline at Month 12 (Per Protocol Set, LOCF)
eTable 3: Treatment Comparison for Change in the Best-Corrected Visual Acuity From Baseline at Month 12 (FAS)
eTable 4: Treatment Comparison for Change in the Best-Corrected Visual Acuity From Baseline at Month 12 (FAS)
eTable 5: Number of Ranibizumab Injections and PDT Treatments Administered Over 12 Months (Safety Set)
eTable 6: Ocular and Nonocular Serious Adverse Events Regardless of Study Drug Relationship Up to Month 12 (safety set)
eTable 7: Ocular and Nonocular Adverse Events Regardless of Study Drug Relationship Up to Month 12 by Preferred Term
References
- 1.Wong RL, Lai TY. Polypoidal choroidal vasculopathy: an update on therapeutic approaches. J Ophthalmic Vis Res. 2013;8(4):359-371. [PMC free article] [PubMed] [Google Scholar]
- 2.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10(1):1-8. [PubMed] [Google Scholar]
- 3.Cheung CM, Laude A, Wong W, et al. . Improved specificity of polypoidal choroidal vasculopathy diagnosis using a modified EVEREST criteria. Retina. 2015;35(7):1375-1380. [DOI] [PubMed] [Google Scholar]
- 4.Kokame GT. Polypoidal choroidal vasculopathy: an important diagnosis to make with therapeutic implications. Retina. 2012;32(8):1446-1448. [DOI] [PubMed] [Google Scholar]
- 5.Khan S, Engelbert M, Imamura Y, Freund KB. Polypoidal choroidal vasculopathy: simultaneous indocyanine green angiography and eye-tracked spectral domain optical coherence tomography findings. Retina. 2012;32(6):1057-1068. [DOI] [PubMed] [Google Scholar]
- 6.Rosa RH Jr, Davis JL, Eifrig CW. Clinicopathologic reports, case reports, and small case series: clinicopathologic correlation of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 2002;120(4):502-508. [DOI] [PubMed] [Google Scholar]
- 7.Laude A, Cackett PD, Vithana EN, et al. . Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29(1):19-29. [DOI] [PubMed] [Google Scholar]
- 8.Cackett P, Yeo I, Cheung CM, et al. . Relationship of smoking and cardiovascular risk factors with polypoidal choroidal vasculopathy and age-related macular degeneration in Chinese persons. Ophthalmology. 2011;118(5):846-852. [DOI] [PubMed] [Google Scholar]
- 9.Cheung CM, Laude A, Yeo I, et al. . Systemic, ocular, and genetic risk factors for age-related macular degeneration and polypoidal choroidal vasculopathy in singaporeans. Sci Rep. 2017;7(7):41386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh AH, Chen LJ, Chen SJ, et al. ; Expert PCV Panel . Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina. 2013;33(4):686-716. [DOI] [PubMed] [Google Scholar]
- 11.Wong CW, Yanagi Y, Lee WK, et al. . Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res. 2016;53:107-139. [DOI] [PubMed] [Google Scholar]
- 12.Tan CS, Ngo WK, Chen JP, Tan NW, Lim TH; EVEREST Study Group . EVEREST study report 2: imaging and grading protocol, and baseline characteristics of a randomised controlled trial of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015;99(5):624-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim TH, Laude A, Tan CS. Polypoidal choroidal vasculopathy: an angiographic discussion. Eye (Lond). 2010;24(3):483-490. [DOI] [PubMed] [Google Scholar]
- 14.Honda S, Matsumiya W, Negi A. Polypoidal choroidal vasculopathy: clinical features and genetic predisposition. Ophthalmologica. 2014;231(2):59-74. [DOI] [PubMed] [Google Scholar]
- 15.Iida T. Polypoidal choroidal vasculopathy with an appearance similar to classic choroidal neovascularisation on fluorescein angiography. Br J Ophthalmol. 2007;91(9):1103-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang K, Si JK, Guo DD, et al. . Ranibizumab alone or in combination with photodynamic therapy vs photodynamic therapy for polypoidal choroidal vasculopathy: a systematic review and Meta-analysis. Int J Ophthalmol. 2015;8(5):1056-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung CM, Lai TY, Chen SJ, et al. . Understanding indocyanine green angiography in polypoidal choroidal vasculopathy: the group experience with digital fundus photography and confocal scanning laser ophthalmoscopy. Retina. 2014;34(12):2397-2406. [DOI] [PubMed] [Google Scholar]
- 18.Tan CS, Ngo WK, Lim LW, Tan NW, Lim TH; EVEREST Study Group . EVEREST study report 3: diagnostic challenges of polypoidal choroidal vasculopathy: lessons learnt from screening failures in the EVEREST study. Graefes Arch Clin Exp Ophthalmol. 2016;254(10):1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh A, Lee WK, Chen LJ, et al. . EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32(8):1453-1464. [DOI] [PubMed] [Google Scholar]
- 20.Oishi A, Kojima H, Mandai M, et al. . Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol. 2013;156(4):644-651. [DOI] [PubMed] [Google Scholar]
- 21.Gomi F, Oshima Y, Mori R, et al. ; Fujisan Study Group . Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: The Fujisan Study. Retina. 2015;35(8):1569-1576. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Zhu A, Egger A, et al. Ranibizumab 0.5 mg in patients with polypoidal choroidal vasculopathy: results from the DRAGON study. Paper presented at: American Academy of Ophthalmology; October 17, 2016; Chicago, IL. [Google Scholar]
- 23.Iida T. Results of the Planet Study. Paper presented at: Asia-Pacific Vitreo-retina Society Annual Meeting; December 9, 2016; Bangkok, Thailand. [Google Scholar]
- 24.Wang W, He M, Zhang X. Combined intravitreal anti-VEGF and photodynamic therapy versus photodynamic monotherapy for polypoidal choroidal vasculopathy: a systematic review and meta-analysis of comparative studies. PLoS One. 2014;9(10):e110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakai T, Okano K, Kohno H, Tsuneoka H. Three-year visual outcomes of intravitreal ranibizumab with or without photodynamic therapy for polypoidal choroidal vasculopathy. Acta Ophthalmol. 2016;94(8):e765-e771. [DOI] [PubMed] [Google Scholar]
- 26.Akaza E, Yuzawa M, Matsumoto Y, Kashiwakura S, Fujita K, Mori R. Role of photodynamic therapy in polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2007;51(4):270-277. [DOI] [PubMed] [Google Scholar]
- 27.Saito M, Nagayama D, Iida T. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy: long-term results. Nippon Ganka Gakkai Zasshi. 2009;113(8):792-799. [PubMed] [Google Scholar]
- 28.Lee WK, Lee PY, Lee SK. Photodynamic therapy for polypoidal choroidal vasculopathy: vaso-occlusive effect on the branching vascular network and origin of recurrence. Jpn J Ophthalmol. 2008;52(2):108-115. [DOI] [PubMed] [Google Scholar]
- 29.Lee YH, Lee EK, Shin KS, Lee KM, Kim JY. Intravitreal ranibizumab combined with verteporfin photodynamic therapy for treating polypoidal choroidal vasculopathy. Retina. 2011;31(7):1287-1293. [DOI] [PubMed] [Google Scholar]
- 30.Hirami Y, Tsujikawa A, Otani A, et al. . Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007;27(3):335-341. [DOI] [PubMed] [Google Scholar]
- 31.Byeon SH, Lew YJ, Lee SC, Kwon OW. Clinical features and follow-up results of pulsating polypoidal choroidal vasculopathy treated with photodynamic therapy. Acta Ophthalmol. 2010;88(6):660-668. [DOI] [PubMed] [Google Scholar]
- 32.Lim JW, Ryu SJ, Shin MC. The effect of intravitreal bevacizumab in patients with acute central serous chorioretinopathy. Korean J Ophthalmol. 2010;24(3):155-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eMethods.
eFigure 1: Retreatment Algorithm
eFigure 2: Categorized Change in BCVA at Month 12 (FAS)
eFigure 3: Proportion of Patients With No Leakage in the Study Eye, by Visit From Months 3 to 12 (FAS)
eFigure 4: Example Case of PCV Patient Treated With Ranibizumab 0.5 mg + vPDT Combination Therapy – Baseline OCT
eFigure 5. Frequency of Ranibizumab Injections Administered Over 12 Months (Safety Set)
eFigure 6: Proportion of Patients With BCVA ≥69 letters at Baseline and Month 12 (FAS)
eTable 1: Treatment Comparison For Change in the Best-Corrected Visual Acuity From Baseline at Month 12 (FAS, LOCF)
eTable 2: Treatment Comparison for Change in the Best-Corrected Visual Acuity From Baseline at Month 12 (Per Protocol Set, LOCF)
eTable 3: Treatment Comparison for Change in the Best-Corrected Visual Acuity From Baseline at Month 12 (FAS)
eTable 4: Treatment Comparison for Change in the Best-Corrected Visual Acuity From Baseline at Month 12 (FAS)
eTable 5: Number of Ranibizumab Injections and PDT Treatments Administered Over 12 Months (Safety Set)
eTable 6: Ocular and Nonocular Serious Adverse Events Regardless of Study Drug Relationship Up to Month 12 (safety set)
eTable 7: Ocular and Nonocular Adverse Events Regardless of Study Drug Relationship Up to Month 12 by Preferred Term