Abstract
Background and Aims
Below-ground carbohydrate storage is considered an adaptation of plants aimed at regeneration after disturbance. A theoretical model by Iwasa and Kubo was empirically tested which predicted (1) that storage of carbohydrates scales allometrically with leaf biomass and (2) when the disturbance regime is relaxed, the ratio of storage to leaf biomass increases, as carbohydrates are not depleted by disturbance.
Methods
These ideas were tested on nine herbaceous species from a temperate meadow and the disturbance regime was manipulated to create recently abandoned and mown plots. Just before mowing in June and at the end of the season in October, plants with below-ground organs were sampled. The material was used to assess the pool of total non-structural carbohydrates and leaf biomass.
Key Results
In half of the cases, a mostly isometric relationship between below-ground carbohydrate storage and leaf biomass in meadow plants was found. The ratio of below-ground carbohydrate storage to leaf biomass did not change when the disturbance regime was less intensive than that for which the plants were adapted.
Conclusions
These findings (isometric scaling relationship between below-ground carbohydrate storage and leaf biomass; no effect of a relaxed disturbance regime) imply that storage in herbs is probably governed by factors other than just the disturbance regime applied once in a growing season.
Keywords: Abandonment, below-ground organs, carbohydrate pool, forbs, disturbance, meadow, mowing, leaf biomass, storage to leaf biomass ratio, TNC
INTRODUCTION
Ecosystems globally are subject to disturbances, and resident plants are adapted to the severity, timing and frequency of disturbances by possessing traits that enable them to avoid, resist and/or tolerate such disturbances. Resistance and avoidance traits are disturbance specific, for example smoke-induced germination (Keeley, 1987) in fire-prone ecosystems or production of specific metabolites under herbivory pressure (Baldwin and Preston, 1999). Tolerance traits, on the other hand, reflect the strategy of how to deal with a wide spectrum of disturbance regimes. Possession of a bud bank and a pool of carbohydrates in below-ground organs out of reach of disturbance, enabling plants to restore biomass lost above-ground, seems to be such a universal tolerance trait (Iwasa and Kubo, 1997; Bellingham and Sparrow, 2000; de Moraes et al., 2016). Paradoxically, while there is profound knowledge of specific adaptations such as thorns, thick bark and thick seed walls, the roles of some tolerance traits in plants, e.g. carbon storage, are still not fully understood. This may be the consequence of the relatively small number of comparative studies carried out in natural communities (e.g. Trlica and Cook, 1971; Palacio et al., 2007; Janeček et al., 2011; Nzunda et al., 2014; Clarke et al., 2016).
From the available data, we know that plants living in a particular ecosystem differ in the amount of carbon stored and, therefore, presumably also in the ability to tolerate disturbance. Interspecific differences are explained by differences in: bud availability (Palacio et al., 2008), taxonomical group (Janeček et al., 2011), shade tolerance (Shibata et al., 2016) and storage compounds (Brocklebank and Hendry, 1989). However, there is no agreement on the question of whether a high concentration of carbohydrates in storage organs results in more vigorous resprouting than a low concentration (Klimeš et al., 1993; Nofal et al., 2004; Bowen and Pate, 2017) or not (Hogg and Lieffers, 1991; Cruz et al., 2003). As the majority of the cited works dealt with the concentration of carbohydrates in storage organs and not with the total carbohydrate pool per plant, the relationship between sprouting ability and carbon storage may be blurred by interspecific differences in the volume of structural vs. storage tissues, which is not related to disturbance tolerance. A few studies examining both the concentration and pool of carbohydrates support this view, since the methods led to different conclusions on the effect of disturbance on carbohydrates and above-ground biomass recovery (Bartoš et al., 2011; Janeček et al., 2015).
In contrast to carbohydrate concentrations, available data on the carbohydrate pool (further also called storage) are very rare and seldom collected for more than a few species from an ecosystem with a specific disturbance regime. The reason is that for the assessment of carbohydrate concentrations, a small fraction of storage tissue from a below-ground organ is sufficient, while for the evaluation of the whole storage tissue we need to excavate an entire organ, which may be impossible in some growth forms and ecosystems.
Due to the lack of empirical data, theoretical concepts on the role of storage in plant strategies remain virtually untested (Bloom et al., 1985; Iwasa and Kubo, 1997; Suzuki and Hutchings, 1997; Suzuki and Stuefer, 1999). Carbohydrate storage is viewed as a way to buffer temporal environmental heterogeneity, including disturbance, seasonal climate variation and different demands for carbohydrates during plant ontogeny/phenology (Martínez-Vilalta et al., 2016). However, the creation of carbohydrate reserves not only provides benefits, because it usually competes with other functions, e.g. growth, maintenance and investment in defence (Chapin et al., 1990; Wiley and Helliker, 2012). The most comprehensive predictions for storage accumulation are provided by a modelling study by Iwasa and Kubo (1997) which predicted the optimal size of storage for recovery after an unpredictable disturbance has removed the entire above-ground biomass.
Iwasa and Kubo (1997) modelled the optimal growth for a theoretical plant having a below-ground storage organ filled with carbohydrates used for regrowth of above-ground organs and flowering. According to the model, plants adapted to a certain level of disturbance suffer from higher mortality when disturbance reaches a higher level, which is due to a shortage of stores for resprouting. In conditions where disturbance is less frequent, plants accumulate more carbohydrates as they are not used for resprouting. For a particular species, a typical ratio between the mass of storage carbohydrates and productive above-ground (leaf) biomass (called S/F by Iwasa and Kubo, 1997) is expected under a specific disturbance regime. Moreover, with increasing age/size of a plant, the storage (pool of stored non-structural carbohydrates) grows faster than the leaf biomass and the S/F is higher in older/larger plants than in smaller/younger plants (Iwasa and Kubo, 1997).
The aim of this study is to test the predictions of Iwasa and Kubo’s model on a temperate meadow subjected to regular removal of above-ground biomass (regular mowing for hay). In accordance with the model, we expected that (1) the amount of storage carbohydrates scaled allometrically with leaf biomass (S/F) and (2) after exclusion of disturbance, the size of storage in relation to leaf biomass (S/F) increased, as carbohydrates will not be depleted by resprouting. We manipulated the disturbance regime, creating recently abandoned and mown plots. Just before mowing in June and at the end of the growing season in October, we sampled only plants for which entire storage organs (roots and/or rhizomes) could be excavated. The material was used to evaluate intraspecific patterns.
MATERIALS AND METHODS
Field experiment
The field experiment was established in two meadows which had been managed for decades as hay meadows in a traditional way, with one harvest at the peak of seasonal development: a dry meadow (Čertoryje in the Bílé Karpaty Mts., Czech Republic, 48°54ʹN, 17°25ʹE, 440 m asl) dominated by the grass Bromus erectus and the graminoid Carex montana (for details, see Klimeš, 1995), and a wet meadow (Ohrazení near České Budějovice, Czech Republic, 48°57ʹN, 14°36ʹE, 500 m asl.) dominated by the tall grass Molinia caerulea (for details, see Lepš, 1999).
In each meadow, we established experimental plots (six in the dry meadow and five in the wet meadow) divided into eight 9 m2 sub-plots in June 2005. Four sub-plots of each plot were mown every year of the experiment (2005–2008) in June, i.e. at the peak of the growing season, when the surrounding meadows also are traditionally harvested (i.e. continuation of the disturbance regime), while four sub-plots were left fallow (change in the disturbance regime). The experimental plots were mown by a hand lawn mower so that 5 cm tall stubble was left. Cut biomass was immediately removed from the plots. We sampled plants twice during the growing season: in June (just before experimental plots were managed) and in October (end of the growing season) in 2006 and 2008. The plants were collected from one mown sub-plot and one abandoned sub-plot of each plot.
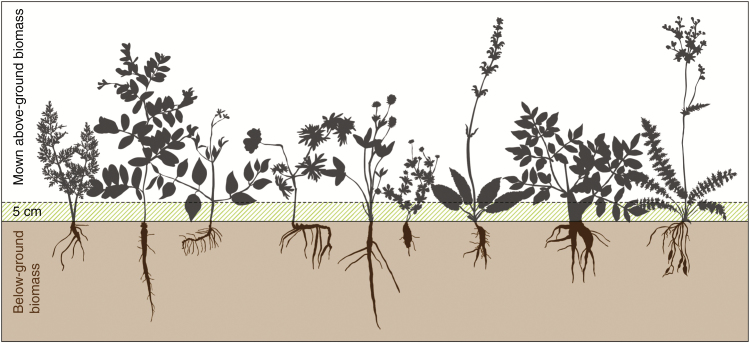
We focused on nine plant species selected for their sufficient abundance and frequency at the start of the experiment and the possibility of digging up their main storage organs completely. We studied six species in the dry meadow (Lathyrus niger, Trifolium montanum, Geranium sanguineum, Salvia pratensis, Clematis recta and Filipendula vulgaris) and three in the wet meadow (Angelica sylvestris, Selinum carvifolia and Potentilla erecta). Schematic drawings of the selected species showing the diversity of below-ground storage organs and differences in removed biomass (for details, see Klimešová et al., 2010; Bartušková et al., 2015) are presented in Fig. 1. At each collection time, one specimen of each plant species from the sampled sub-plot was carefully excavated, soil was immediately removed from its storage organ by brushing and washing, and then the specimen was frozen in liquid nitrogen. The following main carbohydrate storage organs were used for analyses: (1) thick skeletal roots (L. niger, T. montanum, S. pratensis and A. sylvestris); (2) thick skeletal roots + rhizomes (G. sanguineum, C. recta and S. carvifolia); (3) thick roots with root tubers + rhizomes (F. vulgaris); and (4) tuberous rhizomes (P. erecta). Above-ground biomass was separated into three fractions: leaves, stems with petioles, and generative organs, transported to the laboratory and there dried and weighed.
Fig. 1.
Contours of studied species showing the size of their below-ground and above-ground organs. The dashed line denotes a level where biomass is removed by mowing. Species from the left: Selinum carvifolia, Lathyrus niger, Clematis recta, Geranium sanguineum, Trifolium montanum, Potentilla erecta, Salvia pratensis, Angelica sylvestris, and Filipendula vulgaris.
Carbohydrate analyses
The storage organs were freeze-dried and ground. The main storage carbohydrate of the target species is starch, except for S. pratensis, which accumulates raffinose family oligosaccharides (RFOs). For more details on the composition of the carbohydrates in the storage organs of the target species, see Janeček et al. (2011). The starch content was determined using the total starch assay procedure developed by Megazyme International. During this procedure, the starch is first hydrolysed by thermostable α-amylase and amyloglucosidase. The product of hydrolysis (glucose) is subsequently determined calorimetrically with the GOPOD reagent containing glucose oxidase, peroxidase and 4-aminoantipyrine. For more details, see http://www.megazyme.com. Ethanol-soluble carbohydrates, glucose, fructose and sucrose were extracted three times into ethanol, then transferred into water and analysed using a high-performance anion exchange chromatography–pulsed amperometric detector (HPAE-PAD) with a Dionex ICS-3000 system and a CarboPac PA1 analytical column. This analytical system was also used for analyses of RFOs, which are accumulated in storage organs of S. pratensis. RFOs were calculated as the difference between ethanol-soluble carbohydrates (galactose, glucose, fructose and sucrose) before and after addition of α-galactosidase (Aspergillus niger, Megazyme) to the extract. Total non-structural carbohydrates (TNCs) were calculated as the sum of the analysed carbohydrates for each species.
Statistical analyses
Allometric relationships between the TNC pool and leaf biomass were fitted using standardized major axis regressions (SMAs) and tested with the ‘smatr’ package in R (Warton et al., 2012). In contrast to ordinary least squares regression, which minimalizes the errors in the Y direction only, SMA minimizes the sum of squares in both the X and Y dimensions. In consequence, the SMA method is preferable in allometry where both X and Y directions are of the same nature (Warton et al., 2006).
The effects of time (June, October), disturbance (mowing, abandonment) and species identity on the S/F ratio were tested using the permutation mixed effect model, with time and disturbance as fixed factors and species identity as a random factor. Since plant species was used as random factor and the chosen species were different in each meadow, the factor ‘meadow’ became redundant and was not included in the analyses. The reason for including two meadows was to increase the number of species whose storage organs could be dug up completely. We used permutation instead of a parametrical test because we were not able to guarantee the homoscedasticity and normal distribution in the log leaf biomass:log pool of carbohydrates ratio for all plant species. A permutation test rather than a parametric test is also recommended when the number of replicates in individual groups is rather low (Ludbrook and Dudley, 1998). Permutation tests were performed in the PERMANOVA+ program for PRIMER (Anderson et al., 2008).
RESULTS
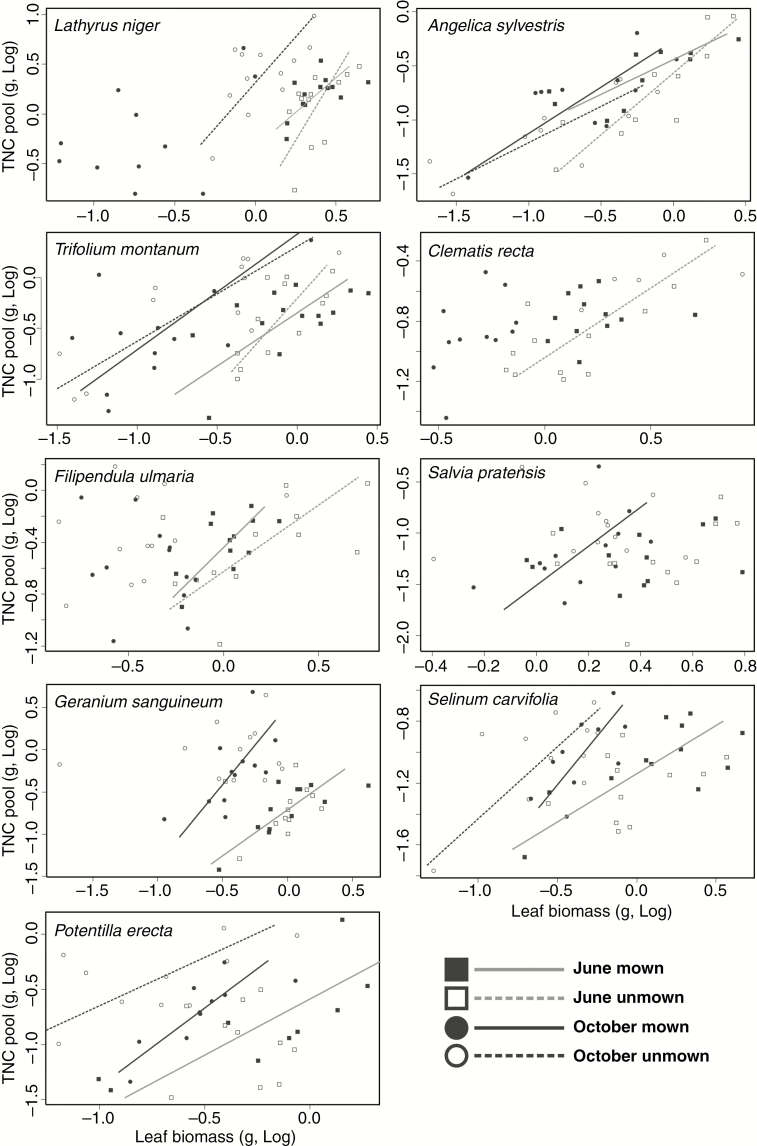
Scaling relationships between the TNC pool and leaf biomass (S/F) at the intraspecific level were found in 50 % of cases and were positive (Table 1; Fig. 2). In most cases (15 out of 18), the slopes did not differ from 1, indicating a prevailing isometric increase in leaf biomass and TNC pool. For four out of nine species (L. niger, T. montanum, G. sanguineum and S. pratensis), we nevertheless recorded slopes which were significantly higher than 1, at least for one Season × Treatment combination (Table 1). However, a joint slope (across all treatments) higher than 1 was found only in the case of L. niger.
Table 1.
Scaling of leaf biomass and carbohydrate TNC pools
| Groups | Lathyrus niger | Trifolium montanum | Filipendula vulgaris | Geranium sanguineum | Potentilla erecta | Angelica sylvestris | Clematis recta | Salvia pratensis | Selinum carvifolia | |
|---|---|---|---|---|---|---|---|---|---|---|
| Correlation (β) | JM | 1.40* | 1.05* | 1.45* | 1.09** | 1.02* | 0.64** | 0.84 | 0.94 | 0.62** |
| JU | 2.69† | 1.76* | 1.02† | 1.38 | -1.82 | 1.14** | 0.93** | 1.53 | 0.68 | |
| OM | 1.19 | 1.14† | -1.59 | 1.87* | 1.44** | 0.86* | 1.82 | 1.92† | 1.26* | |
| OU | 1.84* | 0.93** | 1.09 | 0.67 | 0.88† | 0.67** | 0.46 | –1.20 | 0.93* | |
| CS ≠ 1 (LRS) | 17.34** | 5.47 | 4.25 | 8.14† | 5.18 | 6.70 | 5.98 | 7.15 | 5.93 | |
| Difference of individual slopes from 1 (r) | JM | 0.39 | 0.06 | 0.44 | 0.14 | 0.03 | –0.57† | –0.17 | –0.06 | –0.57† |
| JU | 0.81** | 0.64* | 0.03 | 0.32 | 0.53 | 0.25 | –0.12 | 0.43 | –0.39 | |
| OM | 0.19 | 0.15 | 0.45 | 0.66* | 0.53 | -0.21 | 0.59† | 0.63* | 0.28 | |
| OU | 0.65* | –0.12 | 0.10 | –0.38 | –0.16 | –0.56† | –0.73 | 0.18 | –0.09 | |
| Common slope (β) | 1.70 | 1.14 | 1.26 | 1.21† | 1.21 | 0.83 | 0.98 | 1.39 | 0.82 | |
| JM | – | – | – | AB | – | – | – | – | – | |
| JU | – | – | – | AB | – | – | – | – | – | |
| OM | – | – | – | A | – | – | – | – | – | |
| OU | – | – | – | B | – | – | – | – | – | |
| Shifts in elevation (Wald statistic) | 43.57** | 31.05** | 21.40** | 68.36 | 36.99** | 6.84† | 18.09** | 14.53** | 33.81** | |
| JM | A | A | A | A | A | AB | A | AB | A | |
| JU | A | A | A | A | A | A | A | A | A | |
| OM | B | B | B | B | B | B | B | BD | B | |
| OU | B | B | B | B | C | AB | A | CD | B | |
| Test for shift along the common slope (Wald statistic) | 28.3** | 12.86** | 19.53** | 0.58 | 0.40 | 9.90* | 25.54** | 2.64 | 4.55 | |
| JM | A | A | A | – | – | A | A | – | – | |
| JU | A | A | AB | – | – | A | A | – | – | |
| OM | B | B | C | – | – | AB | B | – | – | |
| OU | A | AB | CB | – | – | B | C | – | – |
Correlation: slopes of individual lines fitted using the standardized major axis approach. Difference of slope from one: statistical tests determining whether the slopes of individual lines differ from β = 1; note that only correlations with P < 0.1 were tested. Common slope: statistical tests determining whether the slopes for individual groups differ from the common slope followed by a post-hoc test in case they differ. Shifts in elevation: tests for plants with a common slope testing whether the groups shift in elevation (i.e. shift along the y-axis) followed by a post-hoc test in case they differ. Test for shifts along the common slope: tests for plants with a common slope testing whether the groups shift along the common slope. Groups: JM, June mown; JU, June unmown; OM, October mown; OU. October unmown.
Groups with the same letters in post-hoc tests do not significantly differ.
CS, common slope; LRS, likelihood ratio statistic; r, sample correlation between residuals and fitted values.
† P < 0.1; *0.05> P >0.01; **P < 0.01.
Fig. 2.
Relationship between leaf biomass and carbohydrate (TNC) pool for individual species sampled on two dates (June and October) and subjected to two treatments (mown and unmown). Note that only regression lines for relationships with P < 0.1 are shown. Related statistical analyses are given in Table 1.
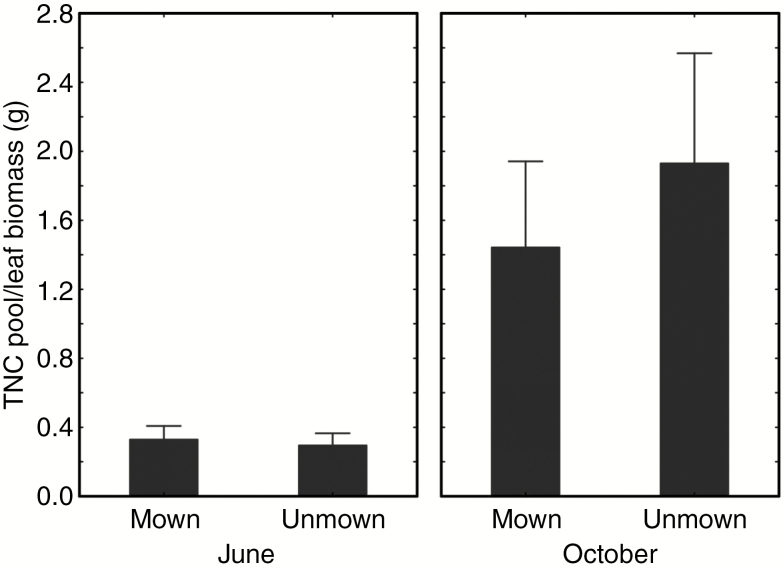
The differences in disturbance treatments and time of sampling manifested themselves in shifts in elevation (eight species), shifts along the joint slope (five species) or both (Table 1). The most obvious differences were between July and October (see post-hoc tests in Table 1) with increased S/F later in the season, due to refilling of TNC stores at the end of the growing season and in some species also due to seasonal leaf loss. The results concerning treatments and species response were similar when we used whole above-ground biomass rather than only biomass of leaves (see Supplementary Data Table S1; Fig. S1).
When testing the effect of sampling time, disturbance and species on the ratio between the carbohydrate pool and leaf biomass, we found that the disturbance, i.e. mowing, had no effect except for interaction with time and species, whereas other explanatory variables separately or in combination affected the ratio significantly (Table 2; Fig. 3). The strongest effect was caused by species identity (Table 2; Fig. 2) which highlights different strategies to cope with environmental perturbations in plants co-occurring in one community.
Table 2.
Effect of time (Ti), disturbance (Di), species (Sp) and their interaction on the ratio between log leaf biomass and log pool of carbohydrates: permutation mixed effect model
| Source | d.f. | Pseudo-F | P(perm) |
|---|---|---|---|
| Time | 1 | 19.397 | 0.0004 |
| Disturbance | 1 | 1.4476 | 0.2579 |
| Species | 8 | 47.194 | 0.0001 |
| Ti × Di | 1 | 4.4139 | 0.045 |
| Ti × Sp | 8 | 4.9513 | 0.0001 |
| Di × Sp | 8 | 2.7471 | 0.0013 |
| Ti × Di × Sp | 8 | 2.8288 | 0.0018 |
| Residuals | 370 | ||
| Total | 405 |
Fig. 3.
Ratio between carbohydrate pool and leaf biomass (S/F ratio) in unmown vs. mown plots in June and October. Means with 1 s.e. are shown.
DISCUSSION
The results imply that we cannot support the predictions by Iwasa and Kubo (1997) that size of below-ground carbohydrate storage generally scales allometrically with leaf biomass and that the ratio of the storage size to leaf biomass increases after exclusion of disturbance. The most frequent relationship found between carbohydrate storage and leaf biomass in meadow plants was isometric and the ratio of storage to leaf biomass did not change when the disturbance regime was less intensive than that to which the plants were presumably adapted.
Isometric scaling relationship between storage and leaf biomass
Iwasa and Kubo expected that plants accumulated carbohydrates disproportionally to their photosynthetic biomass, building an increasingly large storage as they grew, but from our results this does not seem to be the most common scenario in perennial herbs from natural communities.
Overaccumulation of storage may be too costly and disadvantageous, and was also rejected for trees (Palacio et al., 2014). It seems rather that when storage attains a species-specific size, carbohydrates are invested into various plant functions rather than storage (Chapin et al., 1990). In their modelling study, Iwasa and Kubo consider investments into flowering, but also a more vigorous clonal growth (Baptist et al., 2013) and a higher investment into above-ground biomass (Bartušková et al., 2015) have been recorded (see below).
Significant relationships between storage and leaf biomass were scattered among treatments and species. The highest number of significant results were found in June in mown plots, particularly in species forming leaf rosettes and with rare and inconspicuous flowering in meadows, such as A. sylvestris, T. montanum and S. carvifolia (Fig. 1). On the other hand, early and vigorously flowering species such as F. vulgaris and S. pratensis showed a correlation between storage and leaf biomass less often. Those results imply that flowering may substantially affect resource allocation.
Plants differing in size, according to Iwasa and Kubo, just differ in the time available for storage accumulation. However, in real plant communities, plants may be small because they grow in unfavourable spots, e.g. with higher competition by tall neighbours or poor soil. This might have caused noise in our data and could be responsible for a lack of relationship in about half of our analyses. Shaded plants would probably invest more into above-ground biomass than into below-ground biomass and storage, while plants growing in poor soil might accumulate more carbohydrates in comparison with plants growing in rich soil, as their growth is limited by nutrient availability (Bloom et al., 1985; Cruz et al., 2002; Susiluoto et al., 2010; Janeček et al., 2014). We can also speculate that more replicates or focusing only on plants which are either flowering or non-flowering will give more unambiguous results.
Effect of excluded disturbance
We expected, in accordance with Iwasa and Kubo, that storage will increase with excluded disturbance, but this was not the case. The reason may be that plants from abandoned plots invested more into above-ground biomass and flowering (Bartušková et al., 2015), probably at the expense of carbon which should otherwise be invested in regrowth. Despite the higher above-ground biomass of plants from unmown meadows in comparison with mown sites, a higher accumulation of carbohydrates per shoot and higher clonal multiplication in the following year was reported for dominant grasses in unmown vs. mown plots (Bartoš et al., 2011; Baptist et al., 2013)
The role of disturbance in carbohydrate storage may in fact be more complex than we commonly believe. Disturbance just before reproduction can result in higher carbohydrate storage due to saving the carbohydrates which otherwise would have been invested into flowering and fruiting in intact plants. Disturbance at the time of reproduction, on the other hand, may lead to a higher storage exhaustion in comparison with unaffected plants (Sosnová and Klimešová, 2009; Puntieri et al., 2014). Therefore, it would appear that resprouting itself may have a negligible effect on storage due to fast replenishment of storage before the end of the growing season in temperate climates (Wiley et al., 2013; Schmid et al., 2017).
On the other hand, the effect of reproduction and spring regrowth on carbohydrate storage may be lasting a longer time (Zimmerman and Whigham, 1992; Kleijn et al., 2005).
We should also admit that our results may reflect the type of disturbance. Whereas Iwasa and Kubo consider that all above-ground biomass is lost, mowing of a meadow leaves basal parts of the leaves untouched (Fig. 1). Therefore small-stature plants and plants with a basal rosette may preserve part of the leaf biomass from damage (Klimeš and Klimešová, 2001). Consequently, some of the studied species did not have to rely on storage for resprouting only, but might have met their carbon demands by means of compensatory photosynthesis (Wallace et al., 1984; Senock et al., 1991; Thomson et al., 2003).
Further prospects
The results of our study show that we do not understand carbohydrate storage properly and that our theoretical predictions did not match the empirical results. This is in accordance with a recent review by Martínez-Vilalta et al. (2016), concluding that we have not advanced our understanding of plant storage much since the classical reviews by Chapin et al. (1990) and Kozlowski (1992). Despite this, the isometric scaling relationship between carbohydrate storage and leaf biomass, which we found in nearly half of the examined cases, indicates that storage in herbs is probably regulated by sources (biomass of leaves) rather than by sinks (demands due to damage or flowering) (e.g. Millard and Grelet, 2010). This contradicts general expectations (e.g. Iwasa and Kubo, 1997; Dietze et al., 2014). From this point of view, our results may be seen as an encouragement to perform further comparative studies either in natural communities or in pot experiments along disturbance gradients or with manipulated disturbance regimes.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: scaling of above-ground biomass and carbohydrate TNC pools. Figure S1: leaf biomass, TNC pool bivariate plots with lines showing a significant relationship.
Supplementary Material
LITERATURE CITED
- Anderson MJ, Gorley RN, Clarke KR. 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth, UK: PRIMER-E. [Google Scholar]
- Baldwin IT, Preston CA. 1999. The eco-physiological complexity of plant responses to insect herbivores. Planta 208: 137–145. [Google Scholar]
- Baptist F, Viard-Crétat F, Secher-Frommell H et al. 2013. Carbohydrate and nitrogen stores in Festuca paniculata under mowing explain dominance in subalpine grasslands. Plant Biology 15: 395–404. [DOI] [PubMed] [Google Scholar]
- Bartoš M, Janeček Š, Klimešová J. 2011. Effect of mowing and fertilization on biomass and carbohydrate reserves of Molinia caerulea at two organizational levels. Acta Oecologica 37: 299–306. [Google Scholar]
- Bartušková A, Doležal J, Janeček Š, Lanta V, Klimešová J. 2015. Changes in biomass allocation in species rich meadow after abandonment: ecological strategy or allometry?Perspectives in Plant Ecology, Evolution and Systematics 17: 379–397. [Google Scholar]
- Bellingham PJ, Sparrow AD. 2000. Resprouting as a life history strategy in woody plant communities. Oikos 89: 409–416. [Google Scholar]
- Bloom AJ, Chapin FS, Mooney HA. 1985. Resource limitation in plants – an economic analogy. Annual Review of Ecology and Systematics 16: 363–392. [Google Scholar]
- Bowen BJ, Pate JS. 2017. Patterns of storage tissue and starch distribution in the young taproot of obligate seeders and resprouters of Australian Proteaceae (Juss.): possible evidence of homoplastic evolution. Austral Ecology 42: 617–629. [Google Scholar]
- Brocklebank KJ, Hendry GAF. 1989. Characteristics of plant species which store different types of reserve carbohydrates. New Phytologist 112: 255–260. [Google Scholar]
- Chapin FS, Schulze ED, Mooney HA. 1990. The ecology and economics of storage in plants. Annual Review of Ecology and Systematics 21: 423–447. [Google Scholar]
- Clarke PJ, Manea A, Leishman MR. 2016. Are fire resprouters more carbon limited than non-resprouters? Elevated CO2 on biomass, storage and allocation of woody species. Plant Ecology 217: 763–771. [Google Scholar]
- Cruz A, Pérez B, Quintana JR, Moreno JM. 2002. Resprouting in the Mediterranean-type shrub Erica australis affected by soil resource availability. Journal of Vegetation Science 13: 641–650. [Google Scholar]
- Cruz A, Pérez B, Moreno JM. 2003. Plant stored reserves do not drive resprouting of the lignotubers shrub Erica australis. New Phytologist 157: 251–261. [DOI] [PubMed] [Google Scholar]
- Dietze MC, Sala A, Carbone MS et al. 2014. Nonstructural carbon in woody plants. Annual Review of Plant Biology 65: 667–687. [DOI] [PubMed] [Google Scholar]
- Hogg EH, Lieffers VJ. 1991. Seasonal changes in shoot regrowth potential in Calamagrostis canadensis. Oecologia 85: 596–602. [DOI] [PubMed] [Google Scholar]
- Iwasa Y, Kubo T. 1997. Optimal size of storage for recovery after unpredictable disturbances. Evolutionary Ecology 11: 41–65. [Google Scholar]
- Janeček Š, Lanta V, Klimešová J, Doležal J. 2011. Effect of abandonment and plant classification on carbohydrate reserves of meadow plants. Plant Biology 13: 243–251. [DOI] [PubMed] [Google Scholar]
- Janeček Š, Patáčová E, Klimešová J. 2014. Effects of fertilization and competition on plant biomass allocation and internal resources: does Plantago lanceolata follow the rules of economic theory?Folia Geobotanica 49: 49–64. [Google Scholar]
- Janeček Š, Bartušková A, Bartoš M et al. 2015. Effect of disturbance regime on carbohydrate reserves in meadow plants. AoB Plants 7: plv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley JE. 1987. Role of fire in seed germination of woody taxa in California chaparral. Ecology 68: 434–443. [Google Scholar]
- Kleijn D, Treier UA, Müller-Schӓrer HM. 2005. The importance of nitrogen and carbohydrate storage for plant growth of the alpine herb Veratrum album. New Phytologist 166: 565–575. [DOI] [PubMed] [Google Scholar]
- Klimeš L. 1995. Small-scale distribution of species richness in a grassland (Bílé Karpaty Mts., Czech Republic). Folia Geobotanica 30: 499–510. [Google Scholar]
- Klimeš L, Klimešová J. 2001. The effects of mowing and fertilization on carbohydrate reserves and regrowth of grasses: do they promote plant coexistence in species-rich meadows?Evolutionary Ecology 15: 363–382. [Google Scholar]
- Klimeš L, Klimešová J, Osbornová J. 1993. Regeneration capacity and carbohydrate reserves in clonal plant Rumex alpinus: effect of burial. Vegetatio 109: 153–160. [Google Scholar]
- Klimešová J, Janeček Š, Bartušková A, Lanta V, Doležal J. 2010. How is regeneration of plants after mowing affected by shoot size in two species-rich meadows with different water supply?Folia Geobotanica 45: 225–238. [Google Scholar]
- Kozlowski TT. 1992. Carbohydrate sources and sinks in woody plants. Botanical Review 58: 107–222. [Google Scholar]
- Ludbrook J, Dudley H. 1998. Why permutation tests are superior to t and F tests in biomedical research. American Statistican 52: 127–132. [Google Scholar]
- Lepš J. 1999. Nutrient status, disturbance and competition: an experimental test of relationships in a wet meadow. Journal of Vegetation Science 10: 219–230. [Google Scholar]
- Martínez-Vilalta J, Sala A, Asensio D et al. 2016. Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Ecological Monographs 86: 495–516. [Google Scholar]
- Millard P, Grelet GA. 2010. Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiology 30: 1083–1095. [DOI] [PubMed] [Google Scholar]
- de Moraes MG, de Carvalho MAM, Franco AC, Pollock CJ, Figueiredo-Ribeiro RCL. 2016. Fire and drought: soluble carbohydrate storage and survival mechanisms in herbaceous plants from the Cerrado. BioScience 66: 107–117. [Google Scholar]
- Nofal HR, Sosebee RE, Wan C, Borrelli J, Zartman R, Mckenney C. 2004. Mowing right-of-way affects carbohydrate reserves and tiller development. Journal of Range Management 57: 497–502. [Google Scholar]
- Nzunda FE, Griffiths ME, Lawes MJ. 2014. Resource allocation and storage relative to resprouting ability in wind disturbed coastal forest trees. Evolutionary Ecology 28: 735–749. [Google Scholar]
- Palacio S, Maestro M, Montserrat-Martí G. 2007. Relationship between shoot-rooting and root-sprouting abilities and the carbohydrate and nitrogen reserves of Mediterranean dwarf shrubs. Annals of Botany 100: 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio S, Milla R, Albuixech J et al. 2008. Seasonal variability of dry matter content and its relationship with shoot growth and nonstructural carbohydrates. New Phytologist 180: 133–142. [DOI] [PubMed] [Google Scholar]
- Palacio S, Hoch GU, Sala A, Körner C, Millard P. 2014. Does carbon storage limit tree growth?New Phytologist 201: 1096–1100. [DOI] [PubMed] [Google Scholar]
- Puntieri JG, Gatica N, Grosfeld JE. 2014. Flower removal increases rhizome mass in natural populations of Alstroemeria aurea (Alstroemeriaceae). Flora 209: 332–339. [Google Scholar]
- Schmid S, Palacio S, Hoch G. 2017. Growth reduction after defoliation is independent from CO2 supply in deciduous and evergreen young oaks. New Phytologist 214: 1479–490. [DOI] [PubMed] [Google Scholar]
- Senock RS, Sisson WB, Donard GB. 1991. Compensatory photosystesis of Sporobulus flexuosus (Thurb) following simulated herbivory in the northern Chihuahuan desert. Botanical Gazette 152: 275–281. [Google Scholar]
- Shibata R, Kurokawa H, Shibata M et al. 2016. Relationship between resprouting ability, species traits and resource allocation patterns in woody species in a temperate forest. Functional Ecology 30: 1205–1215. [Google Scholar]
- Sosnová M, Klimešová J. 2009. Life-history variation in the short-lived herb Rorippa palustris: the role of carbon storage. Acta Oecologica 35: 691–697. [Google Scholar]
- Susiluoto S, Hilasvuori E, Berninger F. 2010. Testing the growth limitation hypothesis for subarctic Scots pine. Journal of Ecology 98: 1186–1195. [Google Scholar]
- Suzuki JI, Hutchings MJ. 1997. Interactions between shoots in clonal plants and the effects of stored resources on the structure of shoot populations. In: de Kroon H, von Groenendael J, eds. The ecology and evolution of clonal plants. Leiden: Backhuys, 311–329. [Google Scholar]
- Suzuki JI, Stuefer JF. 1999. On the ecological and evolutionary significance of storage in clonal plants. Plant Species Biology 14: 11–17. [Google Scholar]
- Thomson VP, Cunningham SA, Ball MC, Nicotra AB. 2003. Compensation for herbivory by Cucumis sativus through increased photosynthetic capacity and efficiency. Oecologia 134: 167–175. [DOI] [PubMed] [Google Scholar]
- Trlica MJ, Cook CW. 1971. Defoliation effects on carbohydrate reserves of desert species. Journal of Range Management 24: 418–425. [Google Scholar]
- Wallace LL, McNaughton SJ, Coughenour MB. 1984. Compensatory photosynthetic responses of three African graminoids to different fertilization, watering, and clipping regimes. Botanical Gazette 145: 151–156. [Google Scholar]
- Warton DI, Wright IJ, Falster DS, Westoby M. 2006. Bivariate line-fitting methods for allometry. Biological Reviews 81: 259–291. [DOI] [PubMed] [Google Scholar]
- Warton D, Duursma R, Falster D, Taskinen S. 2012. SMATR 3 – an R package for estimation and inference about allometric lines. Methods in Ecology and Evolution 3: 257–259. [Google Scholar]
- Wiley E, Helliker B. 2012. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytologist 195: 285–289. [DOI] [PubMed] [Google Scholar]
- Wiley E, Huepenbecker S, Casper BB, Helliker BR. 2013. The effects of defoliation on carbon allocation: can carbon limitation reduce growth in favour of storage?Tree Physiology 33: 1216–1228. [DOI] [PubMed] [Google Scholar]
- Zimmerman JK, Whigham DF. 1992. Ecological functions of carbohydrates stored in corms of Tipularia discolor (Orchidaceae). Functional Ecology 6: 575–581. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.