Abstract
Surgical strategies for knee joint preservation are numerous, with the procedure(s) of choice for a given patient dependent on the status of the articular cartilage, meniscus, overall alignment, and ligamentous stability. For patients with large, isolated, osteochondral defects of the articular cartilage of the femoral condyle, osteochondral allograft transplantation (OCA) is often performed in an effort to reduce pain and improve function. Similarly, for appropriately indicated patients with symptomatic meniscus deficiency, meniscus allograft transplantation (MAT) is an excellent surgical solution. Often patients require concomitant MAT and OCA as part of a joint preservation strategy. In this Technical Note, we describe the surgical technique for performing arthroscopic-assisted concomitant lateral MAT and lateral femoral condyle OCA as part of a knee joint preservation strategy.
The menisci serve to distribute load and reduce tibiofemoral contact stresses.1 Meniscal tissue has an inherently poor ability to heal given that the blood supply is limited to the peripheral third.2 Meniscus allograft transplantation (MAT) has been shown to be an effective salvage technique to restore meniscal tissue and tibiofemoral biomechanics in the painful, meniscus-deficient knee.3
The long-term effects of meniscal insufficiency including progression to osteoarthritis have been well documented.4, 5, 6 Focal chondral injuries may result from the loss of chondral protection.7 Investigators have reported high graft survivorship and good clinical outcomes of osteochondral allograft transplantation (OCA) as a salvage or primary cartilage restoration procedure in patients with focal chondral defects to restore the articular surface.8, 9 In patients who are meniscal deficient, isolated cartilage restoration is unlikely to provide long-term efficacy. Clinical outcomes of concomitant MAT and OCA have been reported to be similar to outcomes of either procedure done in isolation.8
Investigators have described their technique for isolated lateral MAT (LMAT),10 isolated OCA,11 and concomitant proximal tibia OCA with concomitant LMAT12; however, a paucity of literature exists describing LMAT with OCA to the lateral femoral condyle. In this Technical Note, we describe our technique for combined LMAT with lateral femoral condyle OCA. A summary of the technique is provided in Video 1.
Technique
Surgical Indications
Surgical indications for joint preservation with concomitant lateral MAT and lateral femoral condyle OCA include otherwise healthy patients who are functionally meniscectomized with a concomitant focal articular cartilage defect. This patient population consists predominantly of younger (<50 years old) physically active individuals who are functionally limited by knee pain. Frequently, these patients have failed prior meniscus surgery. Staging arthroscopy is recommended to characterize the extent of the focal cartilage defect and ensure the surrounding cartilage is intact and amenable to OCA. Preoperative radiographs, including long-leg weight-bearing mechanical axis radiographs, are important to confirm neutral alignment.
Patient Positioning and Anesthesia
Following the induction of general anesthesia, the patient was placed in the supine position with the surgical thigh in a leg holder (Fig 1). A well-padded tourniquet was placed high on the thigh. An examination under anesthesia was performed, which revealed full range of motion and stability to ligamentous stress testing. The patient's right lower extremity was prepped and draped in the usual sterile fashion. A time-out was called to confirm the correct patient, procedure, operative site and side, and administration of antibiotics.
Fig 1.
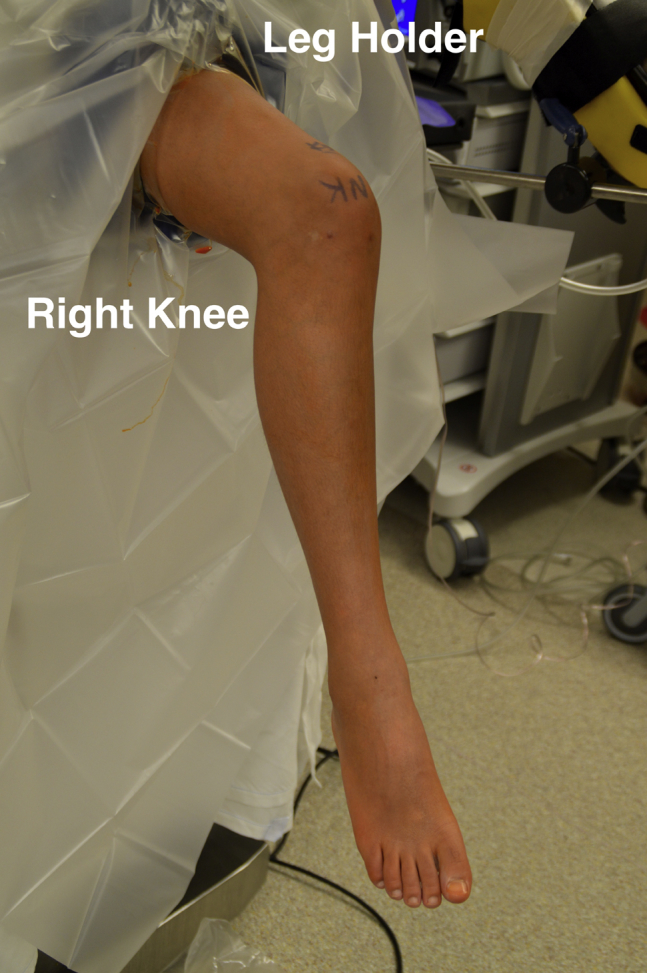
Intraoperative photograph of a patient positioned supine on the operating table with the right leg placed in a standard leg holder. The right knee is prepped and a well-padded thigh tourniquet has been placed.
Surgical Technique—MAT
A diagnostic arthroscopy was performed to confirm the anticipated pathology. Standard inferomedial and inferolateral arthroscopic portals were established. Diagnostic arthroscopy revealed that the medial and patellofemoral compartments were pristine and the cruciate ligaments were intact. Upon entering the lateral compartment, a 15 × 15 mm defect of the lateral femoral condyle in the central weight-bearing zone was identified and debrided. The remaining lateral meniscal tissue was debrided to a peripheral rim of approximately 1 to 2 mm (Fig 2).
Fig 2.
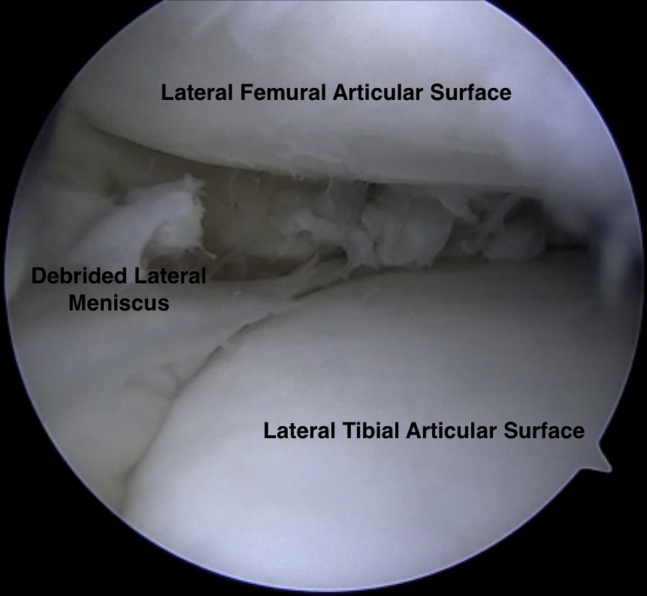
Intraoperative arthroscopic photograph of a supine positioned patient's right knee. The arthroscopic view through the lateral portal demonstrating the lateral meniscus debrided to a peripheral rim of remaining tissue.
Next, beginning at the inferior pole of the patella, a 2-cm longitudinal transpatellar tendon incision was made to allow for MAT using the bridge-in-slot technique. A 4-mm burr was used to make a superficial reference slot, parallel to the sagittal slope of the tibia along the tibial plateau, between the centers of the meniscal horn footprints (Fig 3). A hooked depth gauge (Arthrex, Naples, FL) was inserted at this time to confirm the length of the slot along the tibial plateau followed by placement of a hooked drill guide onto the posterior aspect of the tibial plateau (Fig 4). Next, a guide wire was advanced through the drill guide to, but not through, the posterior cortex (Fig 5). An 8-mm cannulated reamer was introduced over the guide wire to fully establish the slot along the medial aspect of the lateral tibial plateau. An 8 × 10 mm box cutter (Arthrex) was then used to fully establish a 1 cm (deep) by 8 mm (wide) slot to accept the allograft bone bridge. Next a rasp was used to smooth out the edges of the slot (Fig 6).
Fig 3.
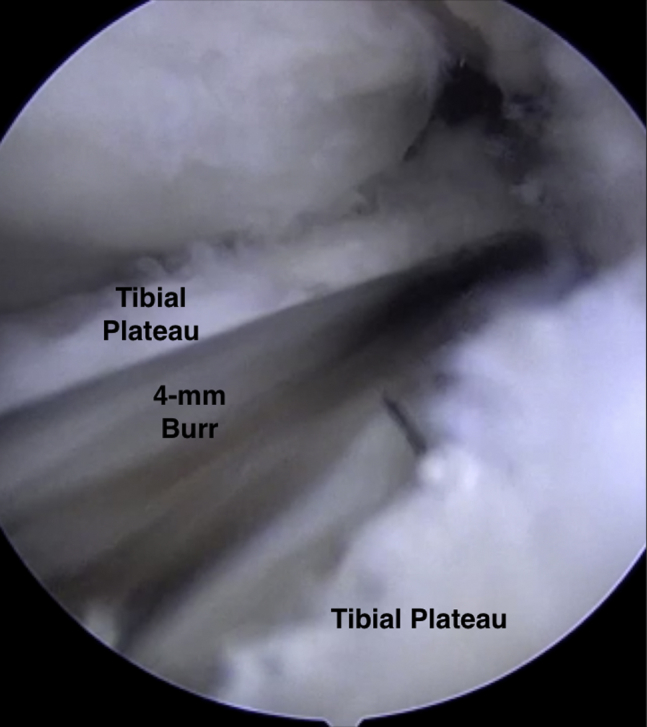
Intraoperative arthroscopic photograph of a supine positioned patient's right knee. The arthroscopic view through the medial portal of the 4-mm burr used to create an index slot along the tibial plateau through which a reamer and box cutter will be used to fully define the slot for the meniscal allograft bone bridge.
Fig 4.
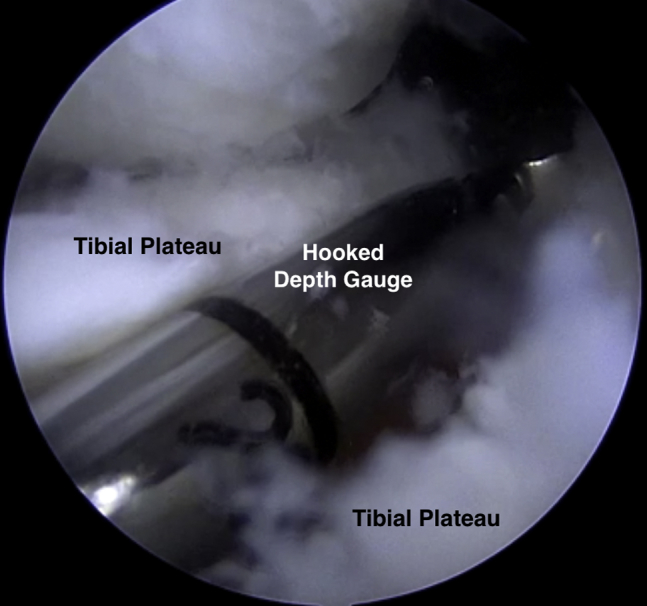
Intraoperative arthroscopic photograph of a supine positioned patient's right knee. The arthroscopic view through the medial portal demonstrating placement of the hooked depth gauge along the reference slot on the lateral tibial plateau.
Fig 5.
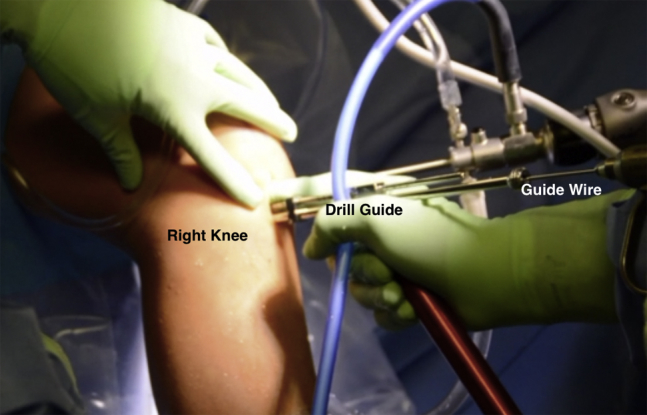
Intraoperative photograph of a patient in the supine position, demonstrating use of the drill guide to place a guide pin along the reference slot in the lateral tibial plateau of the right knee.
Fig 6.
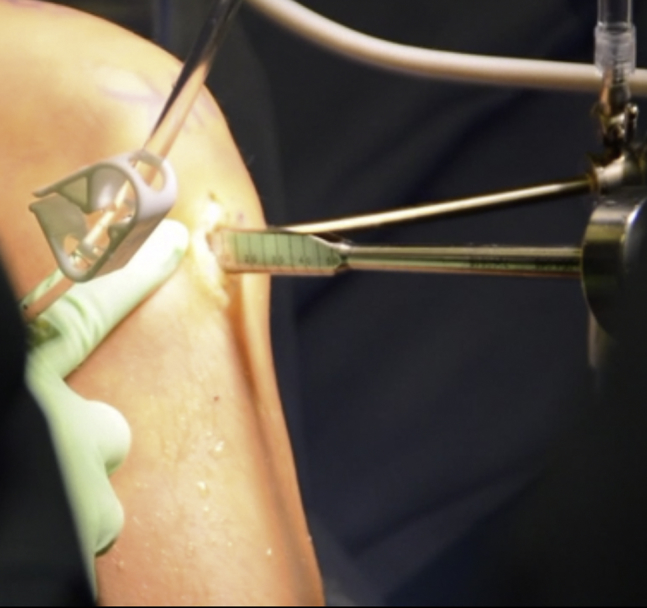
Intraoperative photograph of a supine positioned patient. A large rasp being inserted into the bony slot within the lateral tibial plateau of the right knee. This rasp is used to smooth out the dimensions of the bony slot prior to insertion of the meniscus allograft.
At this time, a posterolateral accessory incision was made approximately 3 cm in length, placed one-third above and two-thirds below the joint line (Fig 7). Following superficial dissection between the iliotibial band and the biceps femoris, the lateral head of the gastrocnemius was elevated off of the posterior capsule, and a Henning retractor (Linvatec, Largo, FL) was placed through the incision (Fig 8).
Fig 7.
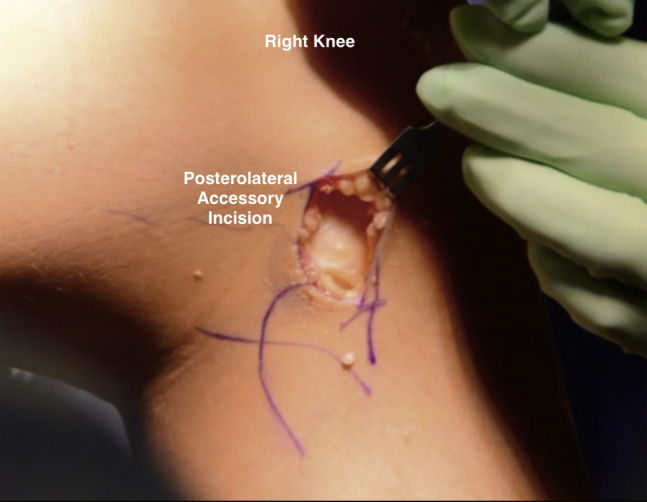
Intraoperative photograph of a patient in the supine position. The photograph depicts the posterolateral accessory incision created on the right knee through which inside-out meniscus repair sutures will be passed to secure the meniscus allograft in place.
Fig 8.
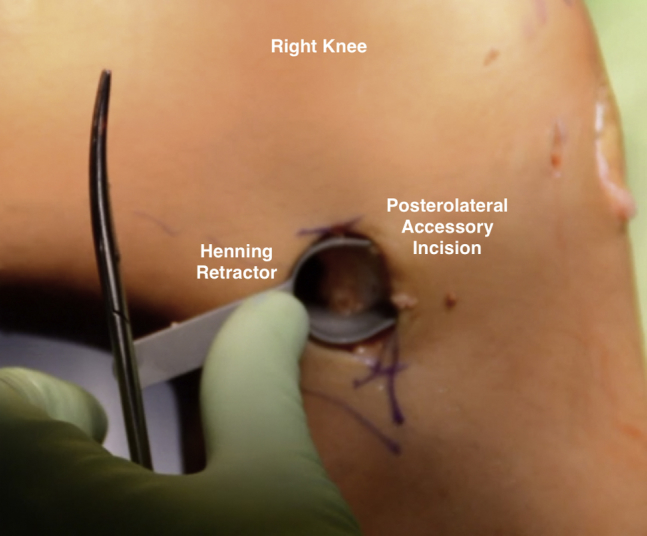
Intraoperative photograph of a patient in the supine position, demonstrating placement of a Henning retractor within the posterolateral accessory incision created on the right knee.
At any point during the case, the meniscus allograft (JRF Ortho, Centennial, CO) can be thawed and prepared on the back table. A sagittal saw was used to prepare the bone bridge to a width of 7 mm and a height of 10 mm to facilitate insertion into the slot. Notably, the bone bridge of the allograft should be undersized by 1 mm to allow easy passage into the slot, which reduces the risk of bone bridge fracture during graft insertion (Fig 9). Care should be taken during graft preparation to avoid injury to the anterior and posterior meniscal attachments on the bone block. The junction of the posterior and middle thirds of the meniscus was marked with a marking pen, and a no. 0 PDS suture (Ethicon, Somerville, NJ) was place in a vertical mattress fashion to be used as a traction suture to facilitate graft passage into the knee (Fig 10).
Fig 9.
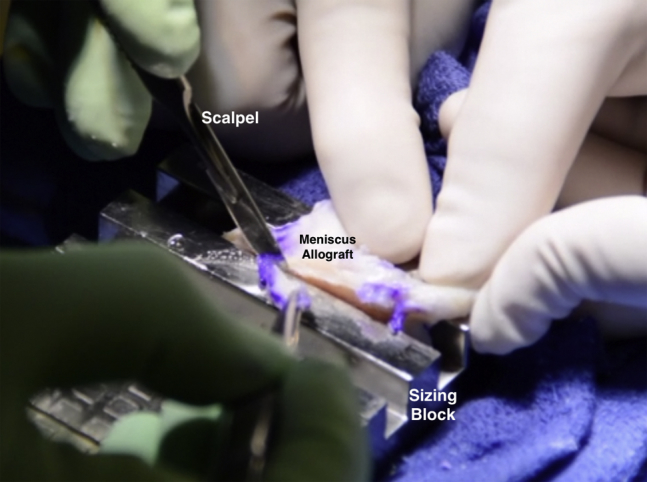
Intraoperative photograph of the meniscal allograft (MAT) being prepared within the meniscus sizing block. A scalpel is used to cut away extraneous tissue adjacent to the bone bridge.
Fig 10.
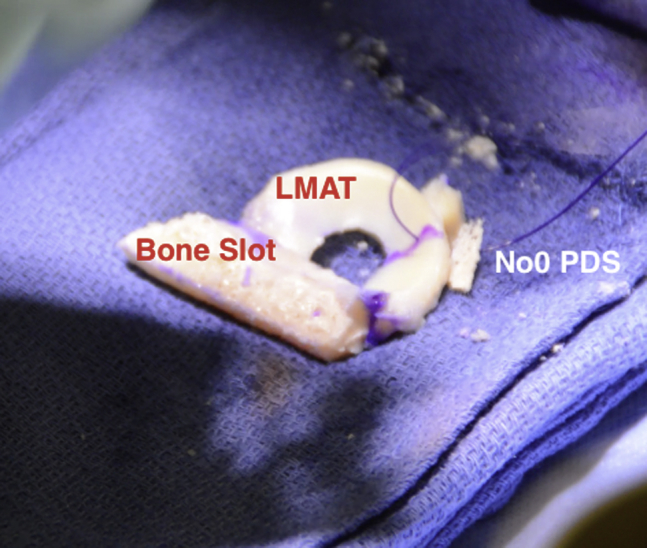
Intraoperative photograph of the lateral meniscal allograft (LMAT). The bony slot and meniscal tissue can be clearly delineated. A no. 0 PDS suture is placed through the junction of the middle and posterior third of the LMAT.
To insert the allograft, the arthroscope was placed in the lateral portal, and a zone-specific meniscal repair cannula was placed through the contralateral portal, directed toward the intended position of the junction of the middle and posterior thirds of the allograft. A flexible nitinol suture-passing wire (Arthrex) was passed through the meniscal repair cannula and withdrawn from posterolateral incision. The looped end of the nitinol wire was then retrieved through the transpatellar tendon incision; the PDS sutures from the allograft were passed through the loop, and the wire and sutures were pulled out of the posterolateral incision. With a gentle pull on PDS traction sutures, the graft was introduced through the transpatellar tendon incision into the joint, taking care to insert the bone bridge into the prepared tibial slot. Once the meniscus was positioned, the knee was gently cycled through flexion/extension to ensure the graft was well positioned under the lateral femoral condyle. At this point, the bone bridge was secured into place with a single 5.75-mm SwiveLock anchor (Arthrex) anteriorly (Fig 11). The remaining graft was then secured to the joint capsule with 10 inside-out vertical mattress meniscus repair sutures placed through zone-specific cannulas, using a standard inside-out meniscus repair technique; notably, the meniscus repair sutures were not tied until the OCA portion of the procedure was complete.
Fig 11.
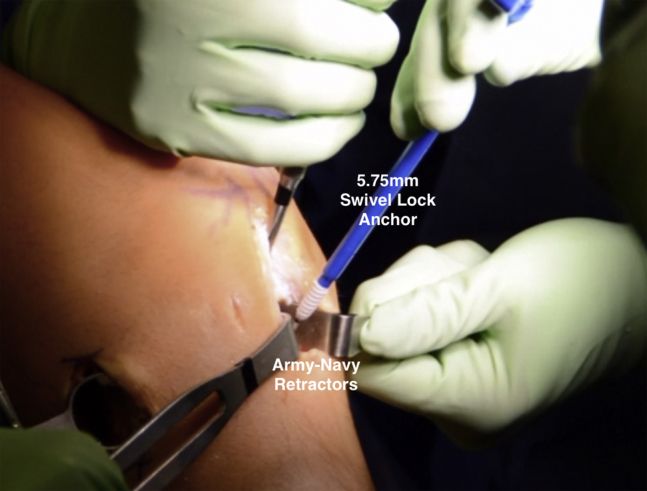
Intraoperative photograph of a patient in the supine position, demonstrating placement of a 5.75-mm SwiveLock anchor into the meniscal allograft bony component to secure it to the right-sided tibia.
Surgical Technique—OCA
The transpatellar tendon incision was extended as needed to facilitate a mini lateral parapatellar arthrotomy for the OCA portion of the procedure. A Z-retractor (Arthrex) was carefully placed in the notch, and the lateral femoral condyle osteochondral defect was identified by flexing the knee. A cannulated cylindrical sizing guide was then used to determine the approximate diameter of the defect, in this case 15 mm (Fig 12). A 2.4-mm guide pin was inserted into the center of the defect through the cylindrical sizing guide (Arthrex). Next the defect was cored with a cannulated circular reamer to approximately 6 to 8 mm in depth and 15 mm in diameter, taking care to use continuous cold irrigation to prevent chondrocyte necrosis (Fig 13). The depth of the reamed defect was then measured at the 3, 6, 9, and 12 o'clock positions to determine appropriate sizing in preparation of the donor graft. The 12 o'clock position was marked on the recipient site to ensure proper alignment when inserting the donor osteochondral plug.
Fig 12.
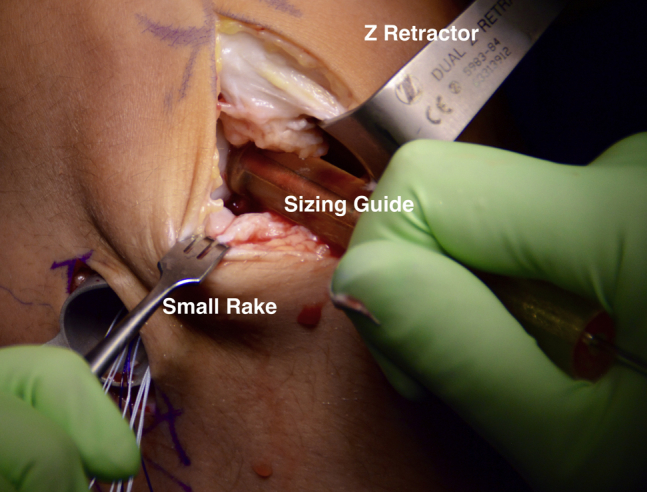
Intraoperative photograph of a patient in the supine position, demonstrating sizing of the lateral femoral condyle osteochondral defect of the right knee using a cannulated cylindrical sizing guide. A Z-retractor and small rack are used to retract the surrounding soft tissue.
Fig 13.
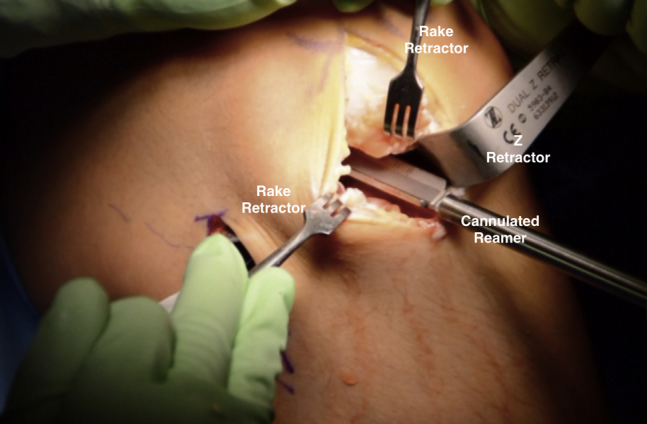
Intraoperative photograph of a patient in the supine position, demonstrating use of a cannulated cutting reamer placed over the previously inserted guide pin into the center of the focal cartilage defect on the lateral femoral condyle of the right knee. This reamer is used to core the defect to a depth of approximately 6 to 8 mm.
Attention was then turned to the back table to prepare the donor hemicondyle (JRF Ortho). A metal bushing (Arthrex) was placed over the area of the donor condyle with approximate contouring to the recipient defect and held by an assistant as the surgeon used a graft harvester (Arthrex) to drill through the entirety of the donor tissue (Fig 14). The osteochondral dowel was removed, and the 12 o'clock position was marked with a marking pen. The dowel was then measured with a ruler and cut down to the appropriate depths based on the measurements previously taken from the recipient defect using a sagittal saw with cold saline irrigation. Pulsatile lavage with 2 L of cold saline was then used to remove any remaining marrow elements from the osteochondral plug (Fig 15). The plug was gently impacted into place with the use of a hand tamp with excellent concentricity and fill after making sure the 12 o'clock marking on the donor graft was aligned with that of the recipient site (Fig 16).
Fig 14.
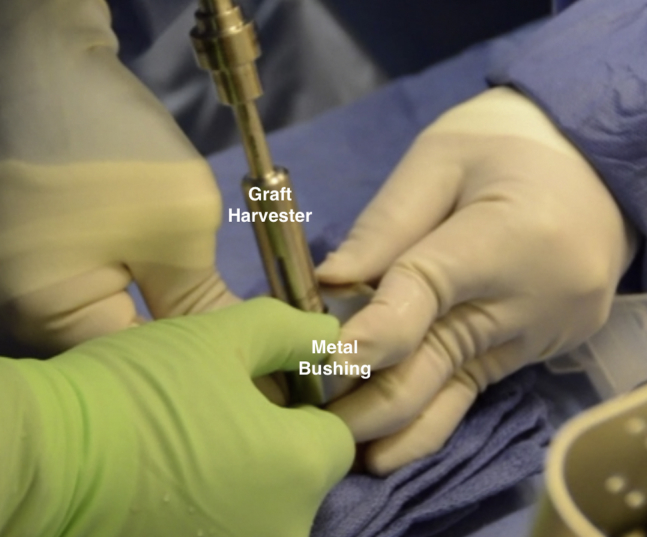
Intraoperative photograph showing an assistant holding a metal bushing with a 15-mm diameter opening over the lateral femoral condyle of a donor hemicondyle while the surgeon uses a graft harvester to drill through the full extent of the hemicondyle to obtain the osteochondral allograft plug.
Fig 15.
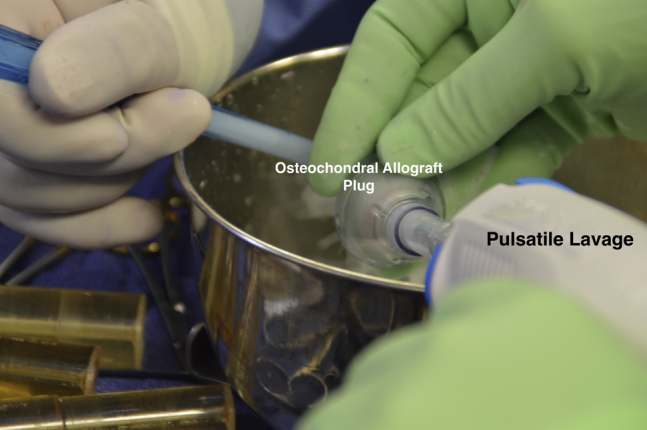
Intraoperative photograph of the osteochondral allograft plug undergoing pulsatile lavage with 2 L of saline to remove any remaining tissue debris and marrow elements.
Fig 16.
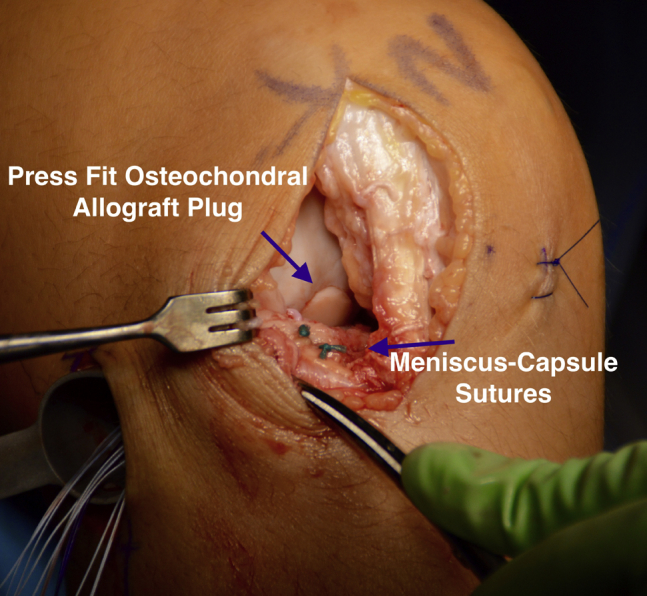
Intraoperative photograph of a patient in the supine position, demonstrating a flush fit of the lateral femoral condyle osteochondral allograft to the surrounding articular cartilage of the right knee. In addition, 2 sutures attaching the anterior aspect of the meniscal allograft to the capsule are visualized.
Meniscal Fixation and Closure
Next the anterior horn of the meniscus was repaired via an outside-in technique (Fig 17) and the inside-out sutures were tied with the knee in full extension. The wounds were copiously irrigated, and the anterior and posterolateral incisions were closed in layers in standard fashion. The portal incisions were closed with interrupted 3-0 nylon sutures (Ethicon). Pearls and pitfalls of the described surgical technique can be found in Table 1, while advantages and disadvantages of this technique may be found in Table 2.
Fig 17.
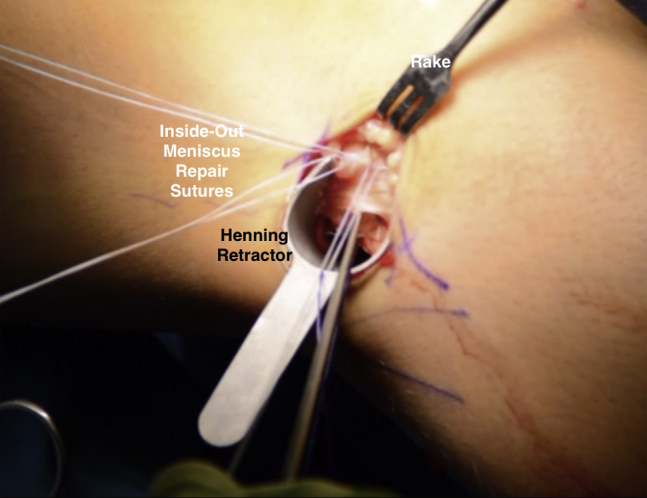
Intraoperative photograph of a patient in the supine position, demonstrating the inside-out meniscus repair sutures being tied down through the posterolateral accessory portal in the right knee. The sutures are evaluated to ensure not to include tissue from the iliotibial band.
Table 1.
Pearls and Pitfalls of the Surgical Technique
| Pearls |
| • Graft mismatch can be avoided through proper graft selection and sizing radiographs. |
| • A spinal needle can be used to localize the ideal placement of the transpatellar incision. |
| • Occasionally, the anterior horn attachment can be larger, up to 9 mm wide. If the anterior horn attachment site is wider than the intended width of the bone bridge, the attachment should be left intact, and the width of the bone bridge should be increased accordingly in the area of the anterior horn insertion only; the remainder of the bone bridge should be trimmed to 7 mm as intended. To accommodate the increased width, the corresponding area of the recipient slot should be widened accordingly. |
| • Undersize the bone bridge of the meniscal allograft by 1 mm to allow easy passage into the slot, which reduces the risk of bone bridge fracture during graft insertion. |
| • It can be helpful to elevate the iliotibial band tissue anteriorly to allow for easier suture tying beneath this structure after the meniscal sutures are passed. |
| • Varus stress can be helpful to open up the lateral compartment of the knee to facilitate graft entry. |
| • All-inside meniscus repair sutures may be helpful to secure the meniscal allograft posteriorly. |
| • Outside-in sutures can be helpful for securing the most anterior aspect of the meniscus. |
| • All instrumentation including sizing, guide pin placement, and reaming of the osteochondral defects must be done perpendicular to produce a graft that matches the recipient site. |
| Pitfalls |
| • Overaggressive reaming along the medial aspect of the tibial plateau can result in iatrogenic injury to the footprint of the anterior cruciate ligament and disruption of the posterior tibial cortex. |
| • Injury to neurovascular structures can occur through inside-out suture placement. |
| • Avoid undersizing the osteochondral allograft. It is better to marginally oversize the osteochondral allograft than leave a marginal quality tissue on the perimeter for the graft to integrate with. |
| • Inaccurate measurements at the 3, 6, 9, and 12 o'clock positions or failure to mark the 12 o'clock position can lead to the graft being recessed or sitting too proud. |
| • Avoid excessive force or a higher number of impactions when placing the osteochondral allograft as that may diminish chondrocyte viability.13, 14 |
Table 2.
Advantages and Disadvantages of the Surgical Technique
| Advantages | Disadvantages |
|---|---|
| • Knee preservation technique restoring the congruity of the previous damaged articular surface with a newly intact meniscus to normalize tibiofemoral contact stressors. | • Technically challenging. |
| • Bridge in slot maintains the proper relationship between the anterior and posterior horns of the lateral meniscus. | • Donor graft tissue not always easily accessible. |
| • Using the bony slot allows easy placement and good osseous integration. | • Expensive procedure for patients. |
Postoperative Rehabilitation
Patients are heal-touch weight bearing for the first 6 weeks with a hinged knee brace locked in extension used for the first 2 weeks. Tibial rotation is avoided for the first 8 weeks to protect the meniscal allograft. Weight bearing is advanced 25% weekly until full beginning at 6 weeks, and full range of motion should be achieved by 8 weeks. Patients may begin use of the stationary bike at 8 weeks and functional activities including walking lunges at 12 weeks. High-impact, sport-specific activities may be advanced beginning at 6 months, but the patient should be cautioned not to return to sport until cleared, typically between 8 and 12 months.
Discussion
While several studies have demonstrated good clinical outcomes of MAT15, 16 and OCA8 performed in isolation, only limited data exist regarding the clinical outcomes of combined procedures for condylar lesions. In a recent publication evaluating the midterm clinical outcomes of 224 consecutive OCAs by a single surgeon, Frank et al.8 reported no significant differences between patients who underwent OCA with MAT compared with those who underwent OCA without MAT. In 2015, Getgood and colleagues17 reported on 48 cases of combined MAT and tibial OCA, 31 of which underwent LMAT, noting a graft survivorship of 78% and 73% for the MAT and OCA at 5 years, respectively. Other researchers have investigated biological knee reconstruction further by evaluating patients who underwent combined osteotomy for malalignment, MAT, and cartilage restoration including OCA, noting significant and clinically meaningful improvements at an average 6.5-year follow-up.18 For combined procedures, MAT is always performed first because of the significant varus stress required for graft passage, placement, and suture fixation.19 Notably, the sutures from the MAT are tied at the end of the procedure, after the OCA has been impacted into place, in order to maintain full knee extension following the procedure.
Lee et al.20 recently compared clinical outcomes and graft survivorship of MAT between patients based on the degree and location of articular cartilage degeneration (Internal Cartilage Repair Society [ICRS] grade ≤2 on both femoral and tibial sides vs ICRS grade 3 or 4 either on the femoral or tibial side vs ICRS grade 3 and 4 on both sides). The investigators noted that while clinical survival rates were not significantly different between groups, on objective evaluation, the estimated 5-year graft survival rate was significantly lower in the high-grade bipolar cartilage lesion group.20 Other investigators have reported that the degree of cartilage wear at the time of MAT is an important predictor of clinical outcome.21
In conclusion, articular cartilage status disease and meniscal deficiency have a symbiotic relationship. If one or the other is in a suboptimal state, it diminishes the clinical efficacy of treating these injuries in isolation. While researchers have shown that these procedures may not be curative,22 treating focal cartilage defects at the time MAT will help maximize the medical benefit of both the cartilage restoration procedure and the MAT.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: B.J.C. receives support from Arthrex, Arthroscopy, Aesculap/B. Braun, American Journal of Orthopedics, American Orthopaedic Society for Sports Medicine, Arthroscopy Association of North America, Athletico, Carticept, Cytori, DJ Orthopaedics, Elsevier Publishing, International Cartilage Repair Society, Journal of Bone and Joint Surgery—American, Journal of Shoulder and Elbow Surgery, Journal of the American Academy of Orthopaedic Surgeons, Medipost, National Institutes of Health (National Institute of Arthritis and Musculoskeletal and Skin Diseases and Eunice Kennedy Shriver National Institute of Child Health and Human Development), Ossur, Regentis, Saunders/Mosby-Elsevier, Smith and Nephew, and Tornier. B.R.W. receives support from Vericel/Genzyme, Arthroscopy, American Journal of Orthopedics, AAOS, AANA, SOMOS, and Elsevier. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Case presentation of a patient undergoing right knee lateral meniscus allograft transplantation with lateral femoral condyle osteochondral allograft transplantation. First, diagnostic arthroscopy is performed to identify anticipated meniscal and chondral pathology. The lateral meniscus is debrided to the vascular edge of the meniscus-capsular junction. Following a 2-cm transpatellar incision, a 4-mm burr is used to make a reference slot along the medial aspect of the lateral tibial plateau. A hooked depth gauged confirms the length of the tibial plateau followed by placement of a hooked drill guide through which a guidewire is advanced. An 8-mm cannulated reamer is introduced to further create a bony slot within the tibial plateau, which is followed by an 8 mm × 10 mm box cutter to fully establish the dimensions of the bony slot. A posterolateral incision is created, and following superficial dissection, a Henning retractor is placed. On the back table, the donor tibia is cut down to isolate the lateral tibial plateau with lateral meniscus. The graft is placed within a meniscal sizing block to ensure appropriate dimensions and subsequently marked at the junction of the middle and posterior third of the meniscus. A no. 0 PDS suture is placed through that junction in a vertical mattress fashion to be used as a traction suture during graft placement. The bone bridge is secured with a single 5.75-mm SwiveLock Anchor. The graft is introduced under arthroscopic visualization and subsequently 8 to 10 inside-out meniscal repair sutures are made. These sutures are not tied until the completion of the osteochondral allograft procedure. A lateral parapatellar arthrotomy is made, and the defect site on the lateral femoral condyle is identified. The lesion is sized using a cylindrical cannulated sizing guide to 15 mm, and a 2.4-mm guide pin is placed into the center of the defect. A cannulated cutting reamer is then placed over the guide pin, and the defect site is reamed to approximately 6 to 8 mm in depth. Measurements are taken with a cut-down ruler at the 3, 6, 9, and 12 o'clock positions. The osteochondral allograft plug is prepared on the back table by placing a metal bushing over the corresponding site on the donor tissue, held by an assistant, while the surgeon uses a graft harvester to obtain the appropriately sized plug. The plug is trimmed to the appropriate depth using a sagittal saw, and the 12 o'clock position is marked. The graft plug undergoes pulsatile lavage with 2 L of saline and is gently impacted with a hand tamp flush to the surrounding cartilage. A suture is placed securing the anterior meniscus to the anterior capsule. The inside-out sutures are tied, and after copious irrigation, the wounds are closed in standard layered fashion.
References
- 1.Messner K., Gao J. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anatomy. 1998;193:161–178. doi: 10.1046/j.1469-7580.1998.19320161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King D. The healing of semilunar cartilages. 1936. Clin Orthop Relat Res. 1990:4–7. [PubMed] [Google Scholar]
- 3.Sohn D.H., Toth A.P. Meniscus transplantation: current concepts. J Knee Surg. 2008;21:163–172. doi: 10.1055/s-0030-1247813. [DOI] [PubMed] [Google Scholar]
- 4.Fairbank T.J. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30:664–670. [PubMed] [Google Scholar]
- 5.Raber D.A., Friederich N.F., Hefti F. Discoid lateral meniscus in children. Long-term follow-up after total meniscectomy. J Bone Joint Surg Am. 1998;80:1579–1586. doi: 10.2106/00004623-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 6.McNicholas M.J., Rowley D.I., McGurty D. Total meniscectomy in adolescence. A thirty-year follow-up. J Bone Joint Surg Br. 2000;82:217–221. [PubMed] [Google Scholar]
- 7.McDermott I.D., Amis A.A. The consequences of meniscectomy. J Bone Joint Surg Br. 2006;88:1549–1556. doi: 10.1302/0301-620X.88B12.18140. [DOI] [PubMed] [Google Scholar]
- 8.Frank R.M., Lee S., Levy D. Osteochondral allograft transplantation of the knee. Am J Sports Med. 2016 doi: 10.1177/0363546516676072. 363546516676072. [DOI] [PubMed] [Google Scholar]
- 9.Briggs D.T., Sadr K.N., Pulido P.A., Bugbee W.D. The use of osteochondral allograft transplantation for primary treatment of cartilage lesions in the knee. Cartilage. 2015;6:203–207. doi: 10.1177/1947603515595072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chahla J., Olivetto J., Dean C.S., Serra Cruz R., LaPrade R.F. Lateral meniscal allograft transplantation: the bone trough technique. Arthrosc Tech. 2016;5:e371–377. doi: 10.1016/j.eats.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean C.S., Chahla J., Serra Cruz R., LaPrade R.F. Fresh osteochondral allograft transplantation for treatment of articular cartilage defects of the knee. Arthrosc Tech. 2016;5:e157–161. doi: 10.1016/j.eats.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Lomas G., Dold A.P., Kaplan D.J., Fralinger D.J., Jazrawi L. Osteochondral proximal tibial and lateral meniscal allograft transplant. Arthrosc Tech. 2016;5:e953–e958. doi: 10.1016/j.eats.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang R.W., Friel N.A., Williams J.M., Cole B.J., Wimmer M.A. Effect of impaction sequence on osteochondral graft damage: the role of repeated and varying loads. Am J Sports Med. 2010;38:105–113. doi: 10.1177/0363546509349038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pylawka T.K., Wimmer M., Cole B.J., Virdi A.S., Williams J.M. Impaction affects cell viability in osteochondral tissues during transplantation. J Knee Surg. 2007;20:105–110. doi: 10.1055/s-0030-1248028. [DOI] [PubMed] [Google Scholar]
- 15.LaPrade R.F., Wills N.J., Spiridonov S.I., Perkinson S. A prospective outcomes study of meniscal allograft transplantation. Am J Sports Med. 2010;38:1804–1812. doi: 10.1177/0363546510368133. [DOI] [PubMed] [Google Scholar]
- 16.McCormick F., Harris J.D., Abrams G.D. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42:892–897. doi: 10.1177/0363546513520115. [DOI] [PubMed] [Google Scholar]
- 17.Getgood A., Gelber J., Gortz S., De Young A., Bugbee W. Combined osteochondral allograft and meniscal allograft transplantation: a survivorship analysis. Knee Surg Sports Traumatol Arthrosc. 2015;23:946–953. doi: 10.1007/s00167-015-3525-8. [DOI] [PubMed] [Google Scholar]
- 18.Harris J.D., Hussey K., Wilson H. Biological knee reconstruction for combined malalignment, meniscal deficiency, and articular cartilage disease. Arthroscopy. 2015;31:275–282. doi: 10.1016/j.arthro.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Harris J.D., Hussey K., Saltzman B.M. Cartilage repair with or without meniscal transplantation and osteotomy for lateral compartment chondral defects of the knee: case series with minimum 2-year follow-up. Orthop J Sports Med. 2014;2 doi: 10.1177/2325967114551528. 2325967114551528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee B.S., Bin S.I., Kim J.M., Kim W.K., Choi J.W. Survivorship after meniscal allograft transplantation according to articular cartilage status. Am J Sports Med. 2017 doi: 10.1177/0363546516682235. 363546516682235. [DOI] [PubMed] [Google Scholar]
- 21.Parkinson B., Smith N., Asplin L., Thompson P., Spalding T. Factors predicting meniscal allograft transplantation failure. Orthop J Sports Med. 2016;4 doi: 10.1177/2325967116663185. 2325967116663185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noyes F.R., Barber-Westin S.D. Long-term survivorship and function of meniscus transplantation. Am J Sports Med. 2016;44:2330–2338. doi: 10.1177/0363546516646375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Case presentation of a patient undergoing right knee lateral meniscus allograft transplantation with lateral femoral condyle osteochondral allograft transplantation. First, diagnostic arthroscopy is performed to identify anticipated meniscal and chondral pathology. The lateral meniscus is debrided to the vascular edge of the meniscus-capsular junction. Following a 2-cm transpatellar incision, a 4-mm burr is used to make a reference slot along the medial aspect of the lateral tibial plateau. A hooked depth gauged confirms the length of the tibial plateau followed by placement of a hooked drill guide through which a guidewire is advanced. An 8-mm cannulated reamer is introduced to further create a bony slot within the tibial plateau, which is followed by an 8 mm × 10 mm box cutter to fully establish the dimensions of the bony slot. A posterolateral incision is created, and following superficial dissection, a Henning retractor is placed. On the back table, the donor tibia is cut down to isolate the lateral tibial plateau with lateral meniscus. The graft is placed within a meniscal sizing block to ensure appropriate dimensions and subsequently marked at the junction of the middle and posterior third of the meniscus. A no. 0 PDS suture is placed through that junction in a vertical mattress fashion to be used as a traction suture during graft placement. The bone bridge is secured with a single 5.75-mm SwiveLock Anchor. The graft is introduced under arthroscopic visualization and subsequently 8 to 10 inside-out meniscal repair sutures are made. These sutures are not tied until the completion of the osteochondral allograft procedure. A lateral parapatellar arthrotomy is made, and the defect site on the lateral femoral condyle is identified. The lesion is sized using a cylindrical cannulated sizing guide to 15 mm, and a 2.4-mm guide pin is placed into the center of the defect. A cannulated cutting reamer is then placed over the guide pin, and the defect site is reamed to approximately 6 to 8 mm in depth. Measurements are taken with a cut-down ruler at the 3, 6, 9, and 12 o'clock positions. The osteochondral allograft plug is prepared on the back table by placing a metal bushing over the corresponding site on the donor tissue, held by an assistant, while the surgeon uses a graft harvester to obtain the appropriately sized plug. The plug is trimmed to the appropriate depth using a sagittal saw, and the 12 o'clock position is marked. The graft plug undergoes pulsatile lavage with 2 L of saline and is gently impacted with a hand tamp flush to the surrounding cartilage. A suture is placed securing the anterior meniscus to the anterior capsule. The inside-out sutures are tied, and after copious irrigation, the wounds are closed in standard layered fashion.


