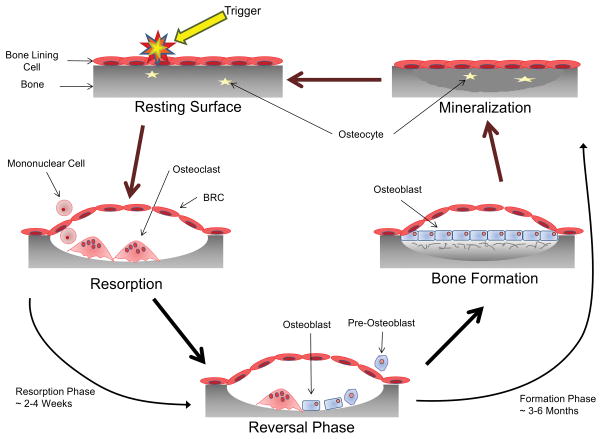
Fig 1. The Bone Remodeling Cycle.
A symplified representation of the bone remodeling cycle. The initiation phase of bone remodeling is induced by mechanical strain, damage or by signals from cytokines or systemic factors. This generates local signals that lead to the bone lining cells separating from the bone surface and forming a canopy over the site to be resorbed (94). Osteoclasts and their precursors are then recruited to the site of bone remodeling from the circulatory system via capillaries that are closely associated with the BRC (95). The signals for the initiation of osteoclast differentiation and resorption; macrophage colony stimulating factor (MCSF) and receptor activator of NF-κB ligand (RANKL), are provided by cells of the osteoblast lineage including osteocytes as well as T and B cells (95–98). Once the remodeling process is initiated resorption of the bone occurs. OC attach to the exposed surface of the mineralized matrix where they polarize and form a sealed microenvironment. This sealed microenvironment is then acidified to breakdown the inorganic component of bone followed by release of the enzymes cathepsin K, matrix metalloproteinase-9 (MMP-9) and tartrate resistant acid phosphatase (TRAP) which breakdown the organic component (7,99). Following resorption of the old damaged bone the process undergoes reversal. Toward the end of the resorption phase of the bone remodeling cycle mononuclear cells of osteoblast-lineage move into the resorption pit. These mononuclear cells remove the old demineralized collagen while laying down a new thin layer (100). During this phase the process of ‘coupling’ bone resorption to bone formation occurs to ensure that the volume of bone removed is replaced. Coupling of bone resorption to bone formation is a multifaceted process with numerous regulator molecules derived from the matrix, secreted or membrane-bound contributing (94,101,102). Bone formation is a two-step process and proceeds slowly, taking approximately 3 months (compared to resorption which typically takes 3 weeks). The osteoblast first secretes the unmineralized osteoid which is then mineralized through the incorporation of hydroxyapatite (103). When the osteoblast has completed the matrix formation they undergo a number of possible fates. The majority of osteoblasts become apoptotic; however, some get trapped in the mineralized matrix and undergo further differentiation into the osteocyte while others may become inactive bone lining cells (104). Through the production of sclerostin (SOST), an inhibitor of Wnt signalling, the osteocyte can regulate the amount of new bone formation that takes place (8).