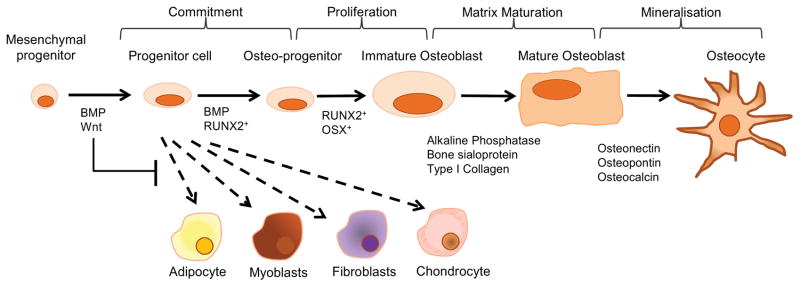
Fig 3. Osteoblast Differentiation.
Signaling by members of the canonical Wnt/β-catenin pathway, such as Wnt10b, and BMP2 and BMP4, direct the MSC cell fate towards the osteoblast lineage. This is achieved by suppressing the adipogenic transcription factors C/EBPα and PPARγ while inducing the osteogenic transcription factors Runx2 and osterix (28,115,116). This immature osteoblast still has the potential to divide and expresses low levels of alkaline phosphatase (ALP) activity, as well as synthesize type I collagen which makes up to 90% of the organic component of bone (117). Differentiation to the non-proliferating mature cuboidal osteoblast that actively mineralizes bone matrix is dependent on the transcription factors osterix (118). Before the newly laid matrix can be mineralized however it must first undergo maturation. Matrix maturation is associated with increased expression of alkaline phosphatase and several non-collagen proteins (NCPs), including osteocalcin, osteopontin, and bone sialoprotein (119). Mineralization of bone is completed by the incorporation of hydroxyapatite (Ca10(PO4)6(OH)2) into the newly deposited osteoid. Membrane bound extracellular bodies (extracellular matrix vesicles) released from the osteoblast facilitate initial mineral deposition by accumulating calcium and phosphate ions in a protected environment. Clusters of these ions come together to form the first stable crystals. Addition of ions to these crystals follows, resulting in their growth (103,120). At the completion of bone formation a subset of osteoblasts can undergo further differentiation, upon being entombed in the bone matrix, and become osteocytes. The remaining osteoblasts are thought to either undergo apoptosis or become inactive bone-lining cells (104).