Abstract
Objective
To evaluate the association of type and timing of prophylactic maternal and infant antiretroviral regimen with time to first positive HIV-1 DNA polymerase chain reaction (PCR) test, in non-breastfed HIV-infected infants from populations infected predominantly with HIV-1 non-B subtype virus.
Design
Analysis of combined data on non-breastfed HIV-infected infants from prospective cohorts in Botswana, Thailand and the United Kingdom (n=405).
Methods
Parametric models appropriate for interval-censored outcomes estimated the time to first positive PCR according to maternal or infant antiretroviral regimen category and timing of maternal antiretroviral initiation, with adjustment for covariates.
Results
Maternal antiretroviral regimens included: no antiretrovirals (n=138), single nucleoside analog reverse transcriptase inhibitor (n=165), single-dose nevirapine with zidovudine (n=66), combination prophylaxis with three or more antiretrovirals (cART, n=36). Type of maternal/infant antiretroviral regimen and timing of maternal antiretroviral initiation were each significantly associated with time to first positive PCR (multivariate p < 0.0001). The probability of a positive test with no antiretrovirals compared with the other regimen/timing groups was significantly lower at one day after birth but did not differ significantly after age 14 days. In a subgroup of 143 infants testing negative at birth, infant cART was significantly associated with longer time to first positive test (multivariate p = 0.04).
Conclusion
Time to first positive HIV-1 DNA-PCR in HIV-1 non-breastfed infants (non-B HIV subtype) may differ according to maternal/infant antiretroviral regimen and may be longer with infant cART, which may have implications for scheduling infant HIV PCR diagnostic testing and confirming final infant HIV status.
Keywords: mother to child transmission of HIV, DNA PCR assays, early infant diagnosis of HIV
Introduction
To assess HIV infection in infants, serologic tests are only reliable when performed beyond 15–18 months of age because infants can carry maternal antibodies for more than a year after birth. In contrast, virologic diagnostic tests that detect the presence of HIV can be used at earlier ages. These tests include viral culture, viral antigen (p24), proviral DNA using polymerase chain reaction (PCR), and HIV DNA/RNA amplification and detection.[1] HIV-1 DNA or RNA amplification assays are recommended for diagnosis of HIV in infants under 15 months of age. Therefore, knowledge of the performance of these assays is essential to inform HIV diagnosis guidelines.
Previous investigations have evaluated the performance characteristics of these virologic assays for the early diagnosis of HIV infection in various individual cohorts.[2–17]. PCR assays typically achieve high specificity, and thus a positive test result is indicative of HIV infection with high probability. However the sensitivity of PCR assays in newborns is lower in the first few weeks of life and increases thereafter. This is likely related to intrapartum transmission, which is not being detected in the newborn sample. For example, in a population of infants infected with subtype B HIV-1, Dunn et al. estimate that 38% (95% CI: [29%, 46%]) of all perinatally infected infants test positive within a day after birth[18]. By 14 days after birth, 93% (95%CI [76%, 97%]) test positive. This heterogeneity in the timing of first positive DNA PCR likely reflects the varying timing of infection (in-utero vs. intrapartum) and the sensitivity of early detection of intrapartum infections (for HIV exposure at labor/delivery, actual establishment of infection with viremia and/or viral DNA integration has not yet occurred). It is also possible that the interplay of elements such as type of antiretroviral prophylaxis regimen, maternal or infant host-mediated factors that may suppress viral replication at delivery, or the sensitivity of different diagnostic assays, may affect detection of virus in the newborn period.
No previous work provides an assessment of the association of combination antiretroviral (ARV) prophylaxis regimen and/or infant prophylaxis with the time to positive signal for DNA assays. Current WHO and other guidelines for clinical management of HIV-infected pregnant women include use of combination ARV regimens.[19–23] Conversely, current recommendations regarding the scheduling of diagnostic tests in HIV-exposed infants are based on studies conducted prior to the era of combination ARV regimens.[24, 25] These prophylactic regimens, being potent suppressors of viral replication, may delay the detection of HIV infection by virus-based assays in infants. Therefore knowledge of the performance of these assays at different infant ages according to type of prophylaxis (particularly maternal and infant combination ARV prophylaxis) is essential to inform HIV diagnosis guidelines.
While several previous studies addressed the performance of HIV-1 DNA assays in HIV-infected infants infected with HIV-1 subtype B virus, relatively few studies have evaluated the performance of HIV-1 DNA assays in infants infected with non-B subtypes [1–3, 5, 10–15]. Non-B subtypes are prevalent in regions such as sub-Saharan Africa and Asia that bear the major burden of mother-to-child transmission of HIV. Previous studies on mother-infant pairs infected with non-B subtype HIV-1 have been limited in sample size, ranging from 38 infants in a study conducted in South Africa[16], where subtype C HIV-1 infection is predominant, to 98 infected infants in a study conducted in Thailand[6], where subtype E HIV-1 or CRF01_AE is most prevalent.
Studying non-breastfed infants provides particular insight regarding the time to positive signal associated with amplification assays, as there is no continuing HIV exposure after birth via breast milk, and timing of transmission is limited to in-utero and intrapartum infection. This paper presents results from the analysis of combined data on HIV-infected, non-breastfed infants and their HIV-infected mothers from prospective studies conducted in three countries in which non-B subtype virus is prevalent.
Methods
Prospective studies of HIV-infected mothers and their non-breastfed infants from Botswana, Thailand and the United Kingdom (UK) were included. All these studies previously received corresponding IRB approvals. Because this study represents a secondary analysis of pooled, de-identified data across the aforementioned studies, the Human Subjects Research Protection offices of the lead institutions involved in the analysis (University of Massachusetts at Amherst and Harvard T. H. Chan School of Public Health) approved this study under a non-human-subjects-research determination. All studies provided individual data on all HIV-infected infants satisfying the following inclusion criteria: (1) At least one HIV-1 DNA PCR result available within three months of birth; (2) Mothers diagnosed with HIV no later than 2 days following delivery; and (3) Infants that were replacement-fed (did not breastfeed).
The dataset included 405 HIV-infected, non-breastfed infants with complete data on maternal and infant ARV regimen. One infant with missing data regarding maternal ARV regimen was excluded from the analysis. All available diagnostic DNA PCR test results for the 405 infants were included in the analysis. A brief description of each included study is provided below, with further details in the Supplemental Digital Content:
Botswana
This 2×2 factorial randomized clinical trial (‘MASHI’) enrolled in Botswana between 2001 and 2003[26], including 91 DNA PCR test results from 32 HIV-infected infants born to mother-infant pairs randomized to the formula-feeding arm of the trial. (Subtype C HIV-1 infection is prevalent).
Thailand (CDC)
Data from two perinatal studies,[7, 27, 28] enrolled in Thailand during 1992–1998 including 370 DNA PCR test results on 122 HIV-infected non-breastfed infants. (Subtype E HIV-1 infection is prevalent among heterosexual women).
Thailand - Program for HIV Prevention and Treatment (PHPT)
Data on 678 DNA PCR test results from 177 HIV-infected non-breastfed infants from two studies conducted in Thailand between 1997 and 2003 were included in this analysis.[29, 30]
United Kingdom - National Study of HIV in Pregnancy and Childhood/Health Protection Agency Collaboration (NSHPC/HPA)
Data comprising 181 DNA PCR test results from 74 HIV-infected non-breastfed infants collected during the period 2000–2009 were analyzed [31–33]. (All mothers acquired HIV in countries with predominantly non-B subtype).
Timing of maternal ARV initiation
The timing of maternal ARV initiation was categorized as follows: (1) No ARV initiated during current pregnancy or at the time of labor/delivery (n=138); (2) ARV initiated during labor/delivery (n=26); (3) ARV initiated during trimester of delivery (n=219); (4) ARV initiated prior to the trimester of delivery (n=22).
Maternal ARV regimen
ARV regimen given to the mother during the trimester closest to delivery and during labor/delivery was used and categorized as: (1) no ARV (n=138); (2) single NRTI (n=165); (3) sdNVP with ZDV (n=66), and (4) three or more ARVs (cART) (n=36).
Infant ARV regimen
Infants prophylactic ARV regimen was categorized as follows: (1) no ARVs (n=143); (2) single NRTI (n=176); (3) sdNVP with ZDV (n=59), and, (4) three or more ARVs (cART) (n=27).
Details regarding the operating definition of HIV infection in infants and other variables are included in the Supplemental Digital Content. The times of DNA PCR tests did not appear to differ by maternal/infant ARV regimen or by time to maternal ARV initiation (see Figures S1–S3 in the Supplemental Digital Content).
Statistical methods
Time to first positive HIV-1 DNA PCR test among all non-breastfed HIV-infected infants was estimated using parametric models appropriate for interval-censored outcomes. The models provide estimates of the probabilities of testing positive by HIV-1 DNA PCR among HIV-infected, non-breastfed infants, by age of infant. Time to first positive HIV-1 DNA PCR was estimated according to maternal or infant ARV regimen category and timing of maternal ARV initiation. These variables could not be modeled jointly due to their high concordance (Table 1). Stratified Weibull models were fit to evaluate the association of each primary variable (maternal/infant ARV regimen, timing of maternal ARV initiation) with time to first positive HIV-1 DNA PCR[34]. Models were adjusted for other covariates including maternal CD4+ cell count and viral load closest to the time of delivery, mode of delivery, gestational age, and infant birth weight. Models could not be adjusted for country because the primary variables were each confounded with country (For example, 94.4% of the subjects in the maternal cART group are from the NSHPC study none were from the studies in Thailand; see Table S1 in the Supplemental Digital Content). As a sensitivity analysis, analyses were repeated for the subgroup 299 HIV-infected infants from the studies in Thailand, to assess whether the results changed when analyses were restricted to one country. Further details on the analyses are included in the Supplemental Digital Content.
Table 1.
Infants classified by Maternal ARV regimen and either infant ARV regimen, or timing of maternal ARV initiation (n=405).
| Infant ARV | Maternal ARV | |||
|---|---|---|---|---|
| No ARV | Single NRTI | sdNVP + ZDV | cART | |
| No ARV | 125 | 18 | 0 | 0 |
| Single NRTI | 4 | 137 | 21 | 14 |
| sdNVP+ZDV | 5 | 8 | 44 | 2 |
| cART | 4 | 2 | 1 | 20 |
| Timing of maternal ARV initiation | ||||
| No ARV | 138 | 0 | 0 | 0 |
| Labor and delivery | 0 | 15 | 10 | 1 |
| During trimester of delivery | 0 | 146 | 53 | 20 |
| Prior to trimester of delivery | 0 | 4 | 3 | 15 |
Abbreviations: Abbreviations: ARV = antiretroviral; cART = combination antiretroviral therapy; NRTI = nucleoside reverse transcriptase inhibitor; sdNVP = single-dose nevirapine; ZDV = zidovudine
Results
The dataset included 405 HIV-infected, non-breastfed infants. Maternal ARV regimen had a high degree of concordance with infant ARV regimen (81%, Table 1). The timing of maternal ARV initiation tended to be earlier in pregnancy for more complex regimens; 42% of women who received cART and 5% or less of women who initiated less complex ARV regimens started prior to the trimester of delivery (Table 1). A similar relationship between infant ARV regimen and timing of maternal ARV initiation was observed (data not shown).
Maternal and infant characteristics according maternal ARV category are shown in Table S1 of the Supplemental Digital Content. The maternal ARV regimen groups differed significantly with respect to several characteristics: Women in the cART group were primarily from the NSHPC cohort and enrolled later, had lower CD4+ cell counts, lower viral loads, were more often diagnosed during pregnancy, and more often had subtype C or mixed subtype than women in the other ARV regimen groups.
While each of the primary variables (maternal/infant ARV, timing of ARV initiation), was significantly associated with time to first positive HIV-1 DNA PCR (p < 0.0001), none of the following maternal and infant characteristics was significant in univariate models: CD4+ cell count closest to delivery (p = 0.24), viral load closest to delivery (p = 0.47), mode of delivery (p = 0.62), gestational age (p = 0.58) and birth weight (p = 0.78).
Maternal ARV regimen and time to first positive HIV-1 DNA PCR
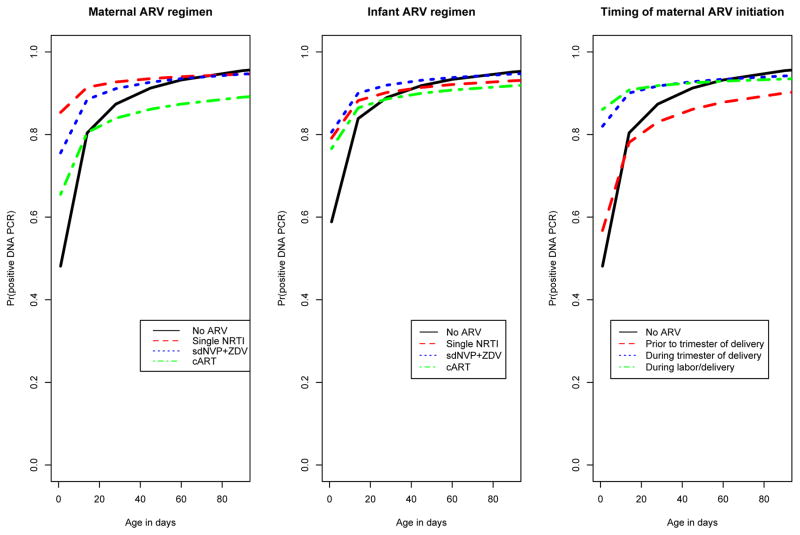
Maternal ARV regimen was significantly associated with time to first positive HIV-1 DNA PCR in a univariate model (p < 0.0001). The probability of a positive HIV-1 DNA PCR test at 1 day after birth (as opposed to later), was significantly lower in the HIV-infected babies in the maternal no ARV group (48% [95% CI: 38%–59%]) when compared to HIV-infected infants in the single NRTI group (85% [95% CI: 80%–90%]) and the sdNVP + ZDV group (76% [95% CI: 65%–85%]). The probability of a positive test at 1 day after birth in the maternal cART group was 66% [95% CI: 49%–81%]. However, the probability of a positive test at or beyond 14 days of age did not differ significantly according to maternal ARV regimen (Table 2, Figure 1a). While overall MTCT rates are lower in infants whose mothers received any ARVs, our analyses were restricted to HIV-infected infants, so the higher probability of a positive test at 1 day after birth among HIV-infected infants whose mothers received any ARV compared with those whose mothers received no ARV may reflect the effect of antenatal and intrapartum treatment with ARVs in reducing intrapartum transmissions; i.e., among HIV-infected infants whose mothers received any ARV, most are in-utero infections (with positive DNA PCR at day 1 after birth) and relatively few are intrapartum infections (with negative DNA PCR at day after birth), while among HIV-infected infants whose mothers received no ARV, both in-utero and intrapartum infections are frequent.
Table 2.
Probabilities of a positive HIV-1 DNA PCR test (95% confidence interval) among HIV-infected non-breastfed infants at 1, 14, 28 and 60 days after birth, according to Maternal ARV regimen; Infant ARV regimen; Timing of maternal ARV initiation (n=405).
| Age at positive DNA PCR test (days since birth) | ||||
|---|---|---|---|---|
|
| ||||
| Maternal ARV category | 1 day | 14 days | 28 days | 60 days |
| Probability | Probability | Probability | Probability | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| No ARV (n=138) | 48% | 80% | 87% | 93% |
| (38%–59%) | (74%–86%) | (82%–92%) | (89%–96%) | |
|
| ||||
| Single NRTI (n=165) | 85% | 91% | 93% | 94% |
| (80%–90%) | (87%–95%) | (89%–96%) | (90%–97%) | |
|
| ||||
| sdNVP + ZDV (n=66) | 76% | 88% | 91% | 94% |
| (65%–85%) | (81%–94%) | (84%–96%) | (87%–97%) | |
|
| ||||
| cART (n=36) | 66% | 80% | 84% | 87% |
| (49%–81%) | (68%–90%) | (72%–93%) | (77%–95%) | |
|
| ||||
| Infant ARV category | ||||
|
| ||||
| No ARV (n=143) | 59% | 84% | 89% | 93% |
| (50%–68%) | (78%–89%) | (84%–93%) | (89%–96%) | |
|
| ||||
| Single NRTI (n=176) | 79% | 88% | 90% | 92% |
| (73%–85%) | (84%–92%) | (86%–94%) | (88%–95%) | |
|
| ||||
| sdNVP + ZDV (n=59) | 81% | 90% | 92% | 94% |
| (70%–89%) | (82%–95%) | (85%–97%) | (87%–98%) | |
|
| ||||
| cART (n=27) | 77% | 86% | 89% | 91% |
| (59%–91%) | (73%–95%) | (76%–96%) | (79%–97%) | |
|
| ||||
| Timing of Maternal ARV Initiation | ||||
|
| ||||
| No ARV (n=138) | 48% | 80% | 87% | 93% |
| (38%–59%) | (74%–86%) | (82%–92%) | (89%–96%) | |
|
| ||||
| Labor and delivery (n=26) | 86% | 91% | 92% | 93% |
| (72%–95%) | (78%–98%) | (79%–98%) | (81%–99%) | |
|
| ||||
| During trimester of delivery (n=219) | 82% | 90% | 92% | 93% |
| (77%–87%) | (86%–93%) | (88%–95%) | (90%–96%) | |
|
| ||||
| Prior to trimester of delivery (n=22) | 57% | 78% | 83% | 88% |
| (37%–78%) | (62%–91%) | (68%–94%) | (74%–96%) | |
Abbreviations: Abbreviations: ARV = antiretroviral; cART = combination antiretroviral therapy; CI = confidence interval; DNA = deoxyribonucleic acid; NRTI = nucleoside reverse transcriptase inhibitor; PCR = polymerase chain reaction; sdNVP = single-dose nevirapine; ZDV = zidovudine
Figure 1.
Probability of a positive HIV-1 DNA PCR test as a function of age (in days) from birth to 90 days among HIV infected infants (n=405), by: (a) Maternal ARV regimen; (b) Infant ARV regimen; (c) Timing of maternal ARV initiation.
In a multivariable model, maternal ARV regimen remained statistically significant after simultaneously adjusting for CD4+ cell count and viral load closest to delivery, mode of delivery, gestational age and birth weight (p < 0.0001). Results were similar when restricted to data from the studies in Thailand (Supplemental Digital Content, Table S3)
Infant ARV regimen and time to first positive HIV-1 DNA PCR
Similar results were obtained from univariate models for the association of infant ARV regimen and time to first positive HIV-1 DNA PCR (Table 2, Figure 1b). In a multivariable model, infant ARV regimen remained statistically significant after simultaneously adjusting for potential confounders listed above (p < 0.0001). Results were similar when restricted to data from the studies in Thailand (Supplemental Digital Content, Table S3)
Timing of maternal ARV initiation and time to first positive HIV- DNA PCR
The timing of maternal ARV initiation was significantly associated with time to first positive HIV-1 DNA PCR in a univariate model (p < 0.0001). The probability of a positive test at 1 day after birth, was significantly lower in the no ARV group (48% [95% CI: 38%–59%]) when compared to infants whose mothers initiated ARVs during the trimester of delivery (82% [95% CI: 77%–87%]) or during labor/delivery (86% [95% CI: 72%–95%]), and did not differ significantly when compared with infants whose mothers initiated ARVs prior to the trimester of delivery (57% [95% CI: 37%–78%]). However, the probability of a positive test at or beyond 14 days of age did not differ significantly according to timing of ARV initiation (Table 2, Figure 1c). As noted above, the higher probability of a positive test at day 1 after birth among HIV-infected infants whose mothers started ARVs either during the antenatal period or during labor and delivery when compared with HIV-infected infants whose mothers received no ARV may reflect the effect of antenatal and intrapartum treatment with ARVs in reducing intrapartum transmissions.
In a multivariable model, timing of maternal ARV initiation remained statistically significant after simultaneously adjusting for CD4+ cell count and viral load closest to delivery, mode of delivery, gestational age and birth weight (p < 0.0001). Results were similar when restricted to data from the studies in Thailand (Supplemental Digital Content, Table S3)
Subgroup analysis of infants testing negative at birth
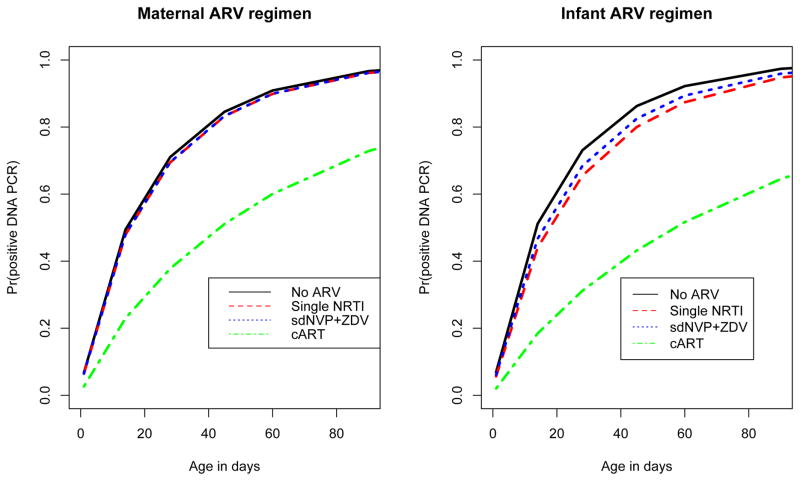
The association of timing and type of maternal/infant ARV regimen with time to first positive DNA PCR may be clearer if the analysis is restricted to a homogeneous subgroup of infants who likely acquired HIV infection during the intrapartum period. In a subgroup analysis of 143 infants who tested negative by DNA PCR within 1 day following birth, maternal ARV regimen type was significantly associated with time to first positive HIV-1 DNA PCR in a univariate model (p =0.02, Figure 2a). However, this association was no longer statistically significant in a multivariable model after adjusting for maternal CD4+ cell count and viral load closest to delivery, mode of delivery, gestational age and birth weight (p =0.09).
Figure 2.
Probability of a positive HIV-1 DNA PCR test as a function of age (in days) from birth to 90 days, among HIV infected infants testing DNA PCR negative at birth (n=143), by: (a) Maternal ARV regimen; (b) Infant ARV regimen.
The association of infant ARV regimen was statistically significant in a univariate model (p=0.04, Figure 2b), and remained significant in a multivariable model after adjusting for maternal CD4+ cell count and viral load closest to delivery, mode of delivery, gestational age and birth weight (p =0.04). By 28 days after birth, the probability of a positive test was 73% [95% CI: 60%–85%] in the No ARV group (n=57), 66% [95% CI: 53% – 77%] in the Single NRTI group (n=68), 68% [95% CI: 44% – 90%] in the sdNVP + ZDV group (n=12), and 31% [95% CI: 14% – 61%] in the cART group (n=6) (Figure 2b). At 60 days after birth, similar patterns across infant ARV regimen categories were observed.
The timing of maternal ARV regimen was not statistically significant in univariate and multivariate models (p > 0.48).
Discussion
Maternal and infant ARV regimen were both significantly associated with time to first positive HIV-1 DNA PCR, in multivariable models after adjusting for potential confounders (p < 0.0001). The probability of a positive test at 1-day of age in the no ARV group was significantly lower when compared to each of the other ARV groups. The timing of maternal ARV initiation was also significantly associated with time to first positive HIV-1 DNA PCR even after adjustment for potential confounders (p < 0.0001). The probability of a positive test at 1 day of age was significantly lower among infants whose mothers received no ARV, when compared to infants whose mothers had ARV initiated during the trimester of delivery or infants whose mothers had ARV initiated during the time of labor/delivery. However, these differences were not statistically significant when infants were tested beyond 2 weeks of age (Table 2). These findings may reflect the prophylactic effects of ARV in reducing the risk of intrapartum transmissions, resulting in a larger proportion of in-utero transmissions in ARV-exposed infants when compared to infants unexposed to ARV[27]. An additional potential explanation for this finding could be a true increase in the sensitivity of the birth PCR for detecting in utero infection, due to a longer period between transmission and testing (if transmission occurred prior to ARV initiation). Whether and to what extent either of the above hypotheses is true cannot be determined by this analysis.
Our results for infants exposed to maternal single NRTI prophylaxis benefit from a larger sample size than in most other studies, for which small sample size might explain the variability in sensitivities found. In our study, the probability of a positive test at 1 day after birth was 85% (95% CI: 80%–88%) among infants whose mothers received single NRTI during pregnancy. Due to small sample sizes ranging from 11–24 infants, previous reports of DNA PCR test positivity rates at birth among infants exposed to single maternal NRTI in populations with subtype B infections have been variable and ranged from 11%–27% [15],[3]. Our findings in the subgroup of infants unexposed to ARV are similar to those reported in the literature in similarly ARV-unexposed infants, infected with subtype B HIV-1. In our study, the probabilities of a positive test by HIV-1 DNA PCR among infants unexposed to ARV were 48% (95% CI: 38%–59%) and 80% (95% CI: 74%–86%) within 1 and 14 days after birth, respectively. Among ARV-unexposed infants infected with subtype B HIV-1, Dunn et al.[18] reported 38% (95% CI: 29%–46%) and 93% (95% CI: 76%–97%) testing positive by DNA PCR at 1 and 14 days after birth, respectively. Other reports among ARV-unexposed infants infected with subtype B HIV-1 concur with these estimates [5, 11–14, 17].
Smaller studies of the sensitivity of DNA PCR for early infant diagnosis of HIV infection have also been conducted in populations infected with non-B subtype virus[6, 7, 16, 17, 35]. Based on 65 infected infants born to HIV-positive mothers enrolled in the French multicenter prospective cohort, Burgard et al. [17] reported that the sensitivity of DNA PCR at birth, 1 month and 3 months were 55%, 89% and 100%, respectively. The results reported in Burgard et al. [17] are consistent with the results in the No ARV group in our study. However, contradictory to our findings, Burgard et al. [17] reported that neither the presence nor type of maternal/infant antiretroviral therapy was significantly associated with time to first positive HIV-1 DNA PCR at birth and at 1 month. Previous reports from two small, prospective studies conducted in South Africa were consistent with the findings of our study - In a prospective cohort of 38 infected infants exposed to at least maternal AZT prophylaxis, sdNVP and infant AZT, the proportions testing positive by HIV-1 DNA PCR at birth and at 4 weeks were 68% (95% CI: 53% – 81%) and 88% (95% CI: 69% – 96%), respectively [16]. In another South African study of 58 infected, non-breastfed infants exposed to sdNVP, Sherman et al. [35] reported that the proportion of HIV infected infants testing positive by HIV-1 DNA PCR at 6 weeks of age was 99%. Data from two previously reported studies conducted in Thailand have been included in our analysis [6, 7].
Our study evaluated the association between timing and type of maternal/infant ARV regimen and time to first positive HIV-1 DNA PCR, in the subgroup of 143 HIV-infected non-breastfed infants who tested DNA PCR negative within 1 day of birth. In this subgroup of infants, all HIV transmissions are likely to have occurred during the intrapartum period. Both maternal and infant ARV regimen had statistically significant associations with time to first positive HIV-1 DNA PCR in univariate models (p = 0.02 and 0.04); the association remained significant in multivariable models for infant ARV but not maternal ARV (p = 0.09 and 0.04). In this subgroup, a longer time to detection of infection by HIV-1 DNA PCR was observed among infants who received cART when compared to infants who received either no ARV or single NRTI. While the sample size in the infant cART group is limited (n=6), these results are consistent with the hypothesis that the lag time between infection and DNA PCR test positivity may be prolonged among infants exposed to highly potent combination antiretroviral regimens when compared to infants unexposed to ARV or those exposed to only monotherapy regimens. These observations may lend support to testing paradigms with more repeat and/or delayed repeat testing among HIV-exposed infants who test negative at birth and are exposed to potent antiretroviral regimens. While our analyses were restricted to data from non-breastfed infants so that all infants had a known time of cessation of exposure to maternal HIV-1 infection, these results equally apply to breastfed infants, suggesting that HIV diagnostic testing scheduled immediately after the end of breastfeeding may be subject to an increased rate of false negatives among infants exposed to cART when compared to infants exposed to less potent ARV regimens. Larger studies of the effects of potent antiretroviral regimens on timing of DNA PCR test positivity are needed.
When infection with nonsubtype B or group O HIV is considered a possibility, it is recommended that both HIV DNA and RNA assays should be performed on infant samples for diagnostic purposes--preferably using DNA and RNA assays that can pick up non clade B or 0 subtypes.[21]. While HIV DNA PCR assays are not as sensitive as HIV RNA assays at detecting nonsubtype B or group O HIV and may have underestimated such infections, HIV RNA assays may be less sensitive than HIV DNA PCR in detecting HIV in the presence of combination ARV drugs[21]. HIV DNA PCR testing was the preferred HIV testing method technology among infants in this study. The most recent World Health Organization infant HIV diagnostic recommendations acknowledge that DNA PCR is currently the standard method for diagnosis of HIV infection in infants[36]. Therefore, although the universe of HIV infected infants in this study may have been underestimated based on our testing methodology, among those who were found to be HIV-infected, HIV DNA PCR testing may have increased the likelihood of detection of HIV infection among infants on combination therapy and the sensitivity of our overall analysis in comparing various perinatal prophylaxis regimens. Therefore, despite a lack of use of RNA testing in this study, we feel that this study provides pertinent results from the point of view of common practice and in more accurately estimating the effect of combination perinatal ARV prophylaxis compared to that of non-combination ARV prophylaxis or no ARV prophylaxis.
Limitations of this analysis include the small number of cART-exposed infants, varying sample collection and handling procedures across studies, and different DNA PCR tests used. Although the analyses were adjusted for potential confounders, there might be residual confounding by study (and by HIV subtype). To address this issue, we repeated the analyses for the subgroup of infants from the studies in Thailand and observed similar trends as in the main analysis (see Tables S2–S3 in the Supplemental Digital Content). Lastly, breastfeeding status was assessed by self-report – however, the possibility of unreported breastfeeding cannot be ruled out definitively.
Earlier testing for all HIV-exposed infants is clearly desirable, as it has been shown that early treatment of infected infants has a significant effect in reducing morbidity and mortality[37, 38]. However, the (very) low rate of HIV transmission with potent ARV prophylaxis might suggest that early testing has limited yield and so repeat testing of almost all infants would be needed[39]. A delayed or additional later repeat testing schedule might be especially important among infants exposed to cART regimens, due to the potent effects of cART regimens on suppressing viral replication. Alternate approaches involving the use of sensitive assays and larger amounts of DNA should increase diagnosis of HIV infection at birth[40]. As the sample size in the cART group was limited in this study, future work evaluating the effects of cART regimens on DNA PCR test positivity in a larger dataset is warranted. Studies among breastfed infants are also crucial in determining the optimal scheduling of testing to facilitate early infant diagnosis of HIV infection[41].
Supplementary Material
Acknowledgments
We gratefully acknowledge the contributions of Professor Stephen Lagakos (Harvard School of Public Health, deceased), whose mentorship and support was invaluable in the conception and development of this study. We would also like to acknowledge the invaluable contribution of Janet Masters, NSHPC data manager and co-ordinator for many years, who was a member of the Collaborative Working Group for this analysis; Janet died in December 2012, tribute at www.nshpc.ucl.ac.uk/nshpc/jmasters.
Author contributions: All authors contributed substantially to the conception, design, and data preparation for this collaborative analysis of pre-existing data. RB, MDH, and DES developed the statistical analysis plan and collaborated on the statistical analyses. RB and DES wrote the first draft of the manuscript and all authors participated in the review and editing of the final manuscript.
NSHPC: National surveillance of obstetric and paediatric HIV in the UK is undertaken through the National Study of HIV in Pregnancy and Childhood (NSHPC) in collaboration with Public Health England, and Health Protection Scotland. The contribution of the midwives, obstetricians, genitourinary physicians, paediatricians, clinical nurse specialists and all other colleagues who report to the NSHPC through the British Paediatric Surveillance Unit of the Royal College of Paediatrics and Child Health, and the obstetric reporting scheme originally established under the auspices of the Royal College of Obstetricians and Gynaecologists, is essential and gratefully acknowledged. The National Study of HIV in Pregnancy and Childhood has Research Ethics Committee approval (Ref: MREC/04/2/009).
MASHI: We thank the following individuals who were instrumental in the design and conduct of the MASHI study: Ibou Thior, MD, MSc; Laura M Smeaton, MSc; Roger L Shapiro, MD, MPH; Carolyn Wester, MD; Lisa Stevens, MD; Trevor Peter, PhD, MPH; Erik van Widenfelt, BSc; Joseph Makhema, MBChB; Kenneth McIntosh, MD; Vladimir Novitsky, MD, PhD; Stephen Lagakos, PhD; Max Essex, PhD.
Thai (CDC) studies: We thank the following individuals for their contributions to the original studies: Dr. Tawee Chotpitayasunondh, Dr. Anuvat Roongpisuthipong, Dr. R.J. Simonds, and Mary Culnane; and Dr. Michelle S. McConnell for her contributions to data extraction and interpretation for this multi-cohort analysis.
Footnotes
Disclaimer: The opinions expressed by authors contributing to this article do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
Conflicts of Interest and Source of Funding: Drs. Balasubramanian, Fowler, Dominguez, and Shapiro were supported by a grant (R21 HD072792) from the National Institutes of Health, USA. Dr. Lockman was supported by funding by the National Institutes of Health R01 HD37793. Dr. Tookey received a grant (GHP/003/013/003) from the Health Protection Agency, UK and from the UK National Screening Programme; the NSHPC is managed within the Population, Policy & Practice Programme at the Institute of Child Health (ICH), University College London (UCL), which previously benefited from funding support from the Medical Research Council (MRC) in its capacity as the MRC Centre of Epidemiology for Child Health (grant number G0400546); the UCL ICH receives a proportion of funding from the UK Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme. Dr Tookey has also received grants from Public Health England, the UK National Screening Programme, the PENTA Foundation, AbbVie Inc, and IATEC/Kendle; she is a member of the Writing Group for the British HIV Association’s Guidelines for the Management of HIV Infection in Pregnant Women. Drs. Huong and Lallemant were supported by grants from The National Institutes of Health, USA (R01 HD 33326; R01 HD 39615). Dr. Tosswill is a member of the Writing Group for the British HIV Association’s Guidelines for the Management of HIV Infection in Pregnant Women. Dr. Palumbo has served as a consultant to Merck Pharmaceuticals and serves on DSMBs for Gilead and ApoPharma. All other authors report no conflicts of interest or sources of funding.
Contributor Information
Raji BALASUBRAMANIAN, Department of Biostatistics and Epidemiology, University of Massachusetts – Amherst, Amherst, MA
Mary Glenn FOWLER, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Kenneth DOMINGUEZ, Medical Epidemiologist, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention.
Shahin LOCKMAN, Brigham and Women’s Hospital, Harvard T. H. Chan School of Public Health, and Botswana Harvard AIDS Institute Partnership (Gaborone, Botswana)
Pat A. TOOKEY, University College Institute of Child Health, 30 Guilford St, London WC1N 1EH, UK
Nicole Ngo Giang HUONG, Institut de recherche pour le développement (IRD) UMI 174 - PHPT, Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand; Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Steven NESHEIM, Centers for Disease Control and Prevention, National Center for HIV, viral Hepatitis, Sexually transmitted disease and Tuberculosis Prevention (NCHHSTP), Division of HIV/AIDS Prevention, Atlanta, GA, USA
Michael D. HUGHES, Department of Biostatistics, Harvard University T. H. Chan School of Public Health, Boston, MA
Marc LALLEMANT, Harvard University T.H. Chan School of Public Health. Boston, MA, USA & PHPT Thailand.
Jennifer TOSSWILL, Virus Reference Department, National Infection Service, Public Health England, London NW9 5EQ, UK
Nathan SHAFFER, Department of HIV/AIDS, World Health Organization1.
Gayle SHERMAN, Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand and National Institute for Communicable Diseases, Johannesburg, South Africa
Paul PALUMBO, Section of Infectious Diseases and International Health, Geisel School of Medicine at Dartmouth, 1 Medical Center Dr., Lebanon, NH 03756
David E. SHAPIRO, Center for Biostatistics in AIDS Research, Harvard University T. H. Chan School of Public Health. Boston, MA, USA
References
- 1.Krivine A, Yakudima A, Le May M, Pena-Cruz V, Huang AS, McIntosh K. A comparative study of virus isolation, polymerase chain reaction, and antigen detection in children of mothers infected with human immunodeficiency virus. J Pediatr. 1990;116(3):372–376. doi: 10.1016/s0022-3476(05)82823-9. [DOI] [PubMed] [Google Scholar]
- 2.Newell ML, Loveday C, Dunn D, Kaye S, Tedder R, Peckham C, De Maria A, Giaquinto C, Omenaca F, Canosa C, et al. Use of polymerase chain reaction and quantitative antibody tests in children born to human immunodeficiency virus-1-infected mothers. J Med Virol. 1995;47(4):330–335. doi: 10.1002/jmv.1890470407. [DOI] [PubMed] [Google Scholar]
- 3.Lambert JS, Harris DR, Stiehm ER, Moye J, Jr, Fowler MG, Meyer WA, 3rd, Bethel J, Mofenson LM. Performance characteristics of HIV-1 culture and HIV-1 DNA and RNA amplification assays for early diagnosis of perinatal HIV-1 infection. J Acquir Immune Defic Syndr. 2003;34(5):512–519. doi: 10.1097/00126334-200312150-00011. [DOI] [PubMed] [Google Scholar]
- 4.Nesheim S, Palumbo P, Sullivan K, Lee F, Vink P, Abrams E, Bulterys M. Quantitative RNA testing for diagnosis of HIV-infected infants. J Acquir Immune Defic Syndr. 2003;32(2):192–195. doi: 10.1097/00126334-200302010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Dunn DT, Simonds RJ, Bulterys M, Kalish LA, Moye J, Jr, de Maria A, Kind C, Rudin C, Denamur E, Krivine A, et al. Interventions to prevent vertical transmission of HIV-1: effect on viral detection rate in early infant samples. AIDS. 2000;14(10):1421–1428. doi: 10.1097/00002030-200007070-00016. [DOI] [PubMed] [Google Scholar]
- 6.Prasitwattanaseree S, Lallemant M, Costagliola D, Jourdain G, Mary JY. Influence of mother and infant zidovudine treatment duration on the age at which HIV infection can be detected by polymerase chain reaction in infants. Antivir Ther. 2004;9(2):179–185. [PubMed] [Google Scholar]
- 7.Young NL, Shaffer N, Chaowanachan T, Chotpitayasunondh T, Vanparapar N, Mock PA, Waranawat N, Chokephaibulkit K, Chuachoowong R, Wasinrapee P, et al. Early diagnosis of HIV-1-infected infants in Thailand using RNA and DNA PCR assays sensitive to non-B subtypes. J Acquir Immune Defic Syndr. 2000;24(5):401–407. doi: 10.1097/00126334-200008150-00001. [DOI] [PubMed] [Google Scholar]
- 8.Sherman GG, Stevens G, Stevens WS. Affordable diagnosis of human immunodeficiency virus infection in infants by p24 antigen detection. Pediatr Infect Dis J. 2004;23(2):173–176. doi: 10.1097/01.inf.0000109332.83246.1a. [DOI] [PubMed] [Google Scholar]
- 9.Sherman GG, Stevens G, Jones SA, Horsfield P, Stevens WS. Dried blood spots improve access to HIV diagnosis and care for infants in low-resource settings. J Acquir Immune Defic Syndr. 2005;38(5):615–617. doi: 10.1097/01.qai.0000143604.71857.5d. [DOI] [PubMed] [Google Scholar]
- 10.Avettand-Fenoel V, Chaix ML, Blanche S, Burgard M, Floch C, Toure K, Allemon MC, Warszawski J, Rouzioux C. LTR real-time PCR for HIV-1 DNA quantitation in blood cells for early diagnosis in infants born to seropositive mothers treated in HAART area (ANRS CO 01) J Med Virol. 2009;81(2):217–223. doi: 10.1002/jmv.21390. [DOI] [PubMed] [Google Scholar]
- 11.Delamare C, Burgard M, Mayaux MJ, Blanche S, Doussin A, Ivanoff S, Chaix ML, Khan C, Rouzioux C. HIV-1 RNA detection in plasma for the diagnosis of infection in neonates. The French Pediatric HIV Infection Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15(2):121–125. doi: 10.1097/00042560-199706010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Steketee RW, Abrams EJ, Thea DM, Brown TM, Lambert G, Orloff S, Weedon J, Bamji M, Schoenbaum EE, Rapier J, et al. Early detection of perinatal human immunodeficiency virus (HIV) type 1 infection using HIV RNA amplification and detection. New York City Perinatal HIV Transmission Collaborative Study. J Infect Dis. 1997;175(3):707–711. doi: 10.1093/infdis/175.3.707. [DOI] [PubMed] [Google Scholar]
- 13.Simonds RJ, Brown TM, Thea DM, Orloff SL, Steketee RW, Lee FK, Palumbo PE, Kalish ML. Sensitivity and specificity of a qualitative RNA detection assay to diagnose HIV infection in young infants. Perinatal AIDS Collaborative Transmission Study. AIDS. 1998;12(12):1545–1549. doi: 10.1097/00002030-199812000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Reisler RB, Thea DM, Pliner V, Green T, Lee F, Nesheim S, Brown T, Kalish M, Folks TM, Heneine W. Early detection of reverse transcriptase activity in plasma of neonates infected with HIV-1: a comparative analysis with RNA-based and DNA-based testing using polymerase chain reaction. J Acquir Immune Defic Syndr. 2001;26(1):93–102. doi: 10.1097/00126334-200101010-00013. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham CK, Charbonneau TT, Song K, Patterson D, Sullivan T, Cummins T, Poiesz B. Comparison of human immunodeficiency virus 1 DNA polymerase chain reaction and qualitative and quantitative RNA polymerase chain reaction in human immunodeficiency virus 1-exposed infants. Pediatr Infect Dis J. 1999;18(1):30–35. doi: 10.1097/00006454-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Lilian RR, Kalk E, Bhowan K, Berrie L, Carmona S, Technau K, Sherman GG. Early diagnosis of in utero and intrapartum HIV infection in infants prior to 6 weeks of age. J Clin Microbiol. 2012;50(7):2373–2377. doi: 10.1128/JCM.00431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgard M, Blanche S, Jasseron C, Descamps P, Allemon MC, Ciraru-Vigneron N, Floch C, Heller-Roussin B, Lachassinne E, Mazy F, et al. Performance of HIV-1 DNA or HIV-1 RNA tests for early diagnosis of perinatal HIV-1 infection during anti-retroviral prophylaxis. J Pediatr. 2012;160(1):60–66. e61. doi: 10.1016/j.jpeds.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 18.Dunn DT, Brandt CD, Krivine A, Cassol SA, Roques P, Borkowsky W, De Rossi A, Denamur E, Ehrnst A, Loveday C. The sensitivity of HIV-1 DNA polymerase chain reaction in the neonatal period and the relative contributions of intra-uterine and intra-partum transmission. AIDS. 1995;9(9):F7–11. doi: 10.1097/00002030-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Jamieson DJ, Clark J, Kourtis AP, Taylor AW, Lampe MA, Fowler MG, Mofenson LM. Recommendations for human immunodeficiency virus screening, prophylaxis, and treatment for pregnant women in the United States. Am J Obstet Gynecol. 2007;197(3 Suppl):S26–32. doi: 10.1016/j.ajog.2007.03.087. [DOI] [PubMed] [Google Scholar]
- 20.Buchanan AM, Cunningham CK. Advances and failures in preventing perinatal human immunodeficiency virus infection. Clin Microbiol Rev. 2009;22(3):493–507. doi: 10.1128/CMR.00054-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. [ aidsinfo.nih.gov/contentfiles/lvguidelines/perinatalgl.pdf]
- 22.Consolidated Guidelines on The Use of Antiretroviral Drugs For Treating and Preventing HIV Infection - Recommendations for a public health approach. [ http://www.who.int/entity/hiv/pub/guidelines/keypopulations/en/index.html]
- 23.Current guidelines: British HIV Association (BHIVA) [ http://www.bhiva.org/guidelines.aspx]
- 24.Read JS. Diagnosis of HIV-1 infection in children younger than 18 months in the United States. Pediatrics. 2007;120(6):e1547–1562. doi: 10.1542/peds.2007-2951. [DOI] [PubMed] [Google Scholar]
- 25.WHO. March 2014 Supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2014 [Google Scholar]
- 26.Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, Gilbert PB, Stevens L, Peter T, Kim S, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296(7):794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 27.Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, Chotpitayasunondh T, Chearskul S, Roongpisuthipong A, Chinayon P, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353(9155):773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 28.Shaffer N, Roongpisuthipong A, Siriwasin W, Chotpitayasunondh T, Chearskul S, Young NL, Parekh B, Mock PA, Bhadrakom C, Chinayon P, et al. Maternal virus load and perinatal human immunodeficiency virus type 1 subtype E transmission, Thailand. Bangkok Collaborative Perinatal HIV Transmission Study Group. J Infect Dis. 1999;179(3):590–599. doi: 10.1086/314641. [DOI] [PubMed] [Google Scholar]
- 29.Lallemant M, Jourdain G, Le Coeur S, Kim S, Koetsawang S, Comeau AM, Phoolcharoen W, Essex M, McIntosh K, Vithayasai V. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. Perinatal HIV Prevention Trial (Thailand) Investigators. N Engl J Med. 2000;343(14):982–991. doi: 10.1056/NEJM200010053431401. [DOI] [PubMed] [Google Scholar]
- 30.Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, Koetsawang S, Kanshana S, McIntosh K, Thaineua V. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351(3):217–228. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 31.Judd A, Ferrand RA, Jungmann E, Foster C, Masters J, Rice B, Lyall H, Tookey PA, Prime K. Vertically acquired HIV diagnosed in adolescence and early adulthood in the United Kingdom and Ireland: findings from national surveillance. HIV Med. 2009;10(4):253–256. doi: 10.1111/j.1468-1293.2008.00676.x. [DOI] [PubMed] [Google Scholar]
- 32.Townsend CL, Byrne L, Cortina-Borja M, Thorne C, de Ruiter A, Lyall H, Taylor GP, Peckham CS, Tookey PA. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS. 2014;28(7):1049–1057. doi: 10.1097/QAD.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 33.Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS. 2008;22(8):973–981. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 34.Gu X, Shapiro D, Hughes MD, Balasubramanian R. Stratified Weibull Regression Model for Interval-Censored Data. R Journal. 2014;6(1):10. [PMC free article] [PubMed] [Google Scholar]
- 35.Sherman GG, Cooper PA, Coovadia AH, Puren AJ, Jones SA, Mokhachane M, Bolton KD. Polymerase chain reaction for diagnosis of human immunodeficiency virus infection in infancy in low resource settings. Pediatr Infect Dis J. 2005;24(11):993–997. doi: 10.1097/01.inf.0000187036.73539.8d. [DOI] [PubMed] [Google Scholar]
- 36.WHO. WHO Recommendations on the Diagnosis of HIV Infection in Infants and Children. World Health Organization; 2010. Available at: http://www.who.int/hiv/pub/paediatric/diagnosis/en/ [PubMed] [Google Scholar]
- 37.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, McIntyre JA. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lilian RR, Kalk E, Technau KG, Sherman GG. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr Infect Dis J. 2013;32(10):1080–1085. doi: 10.1097/INF.0b013e318290622e. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, Makhema J, Moyo S, Thior I, McIntosh K, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362(24):2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell C, Dross S, Beck IA, Micek MA, Frenkel LM. Low concentrations of HIV-1 DNA at birth delays diagnosis, complicating identification of infants for antiretroviral therapy to potentially prevent the establishment of viral reservoirs. Clin Infect Dis. 2014;58(8):1190–1193. doi: 10.1093/cid/ciu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King CC, Kourtis AP, Persaud D, Nelson JA, Ziemniak C, Hudgens MG, Tegha G, Chasela CS, Jamieson DJ, van der Horst CM. Delayed HIV detection among infants exposed to postnatal antiretroviral prophylaxis during breastfeeding. AIDS. 2015 Sep 24;29(15):1953–61. doi: 10.1097/QAD.0000000000000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.