Figure 1.
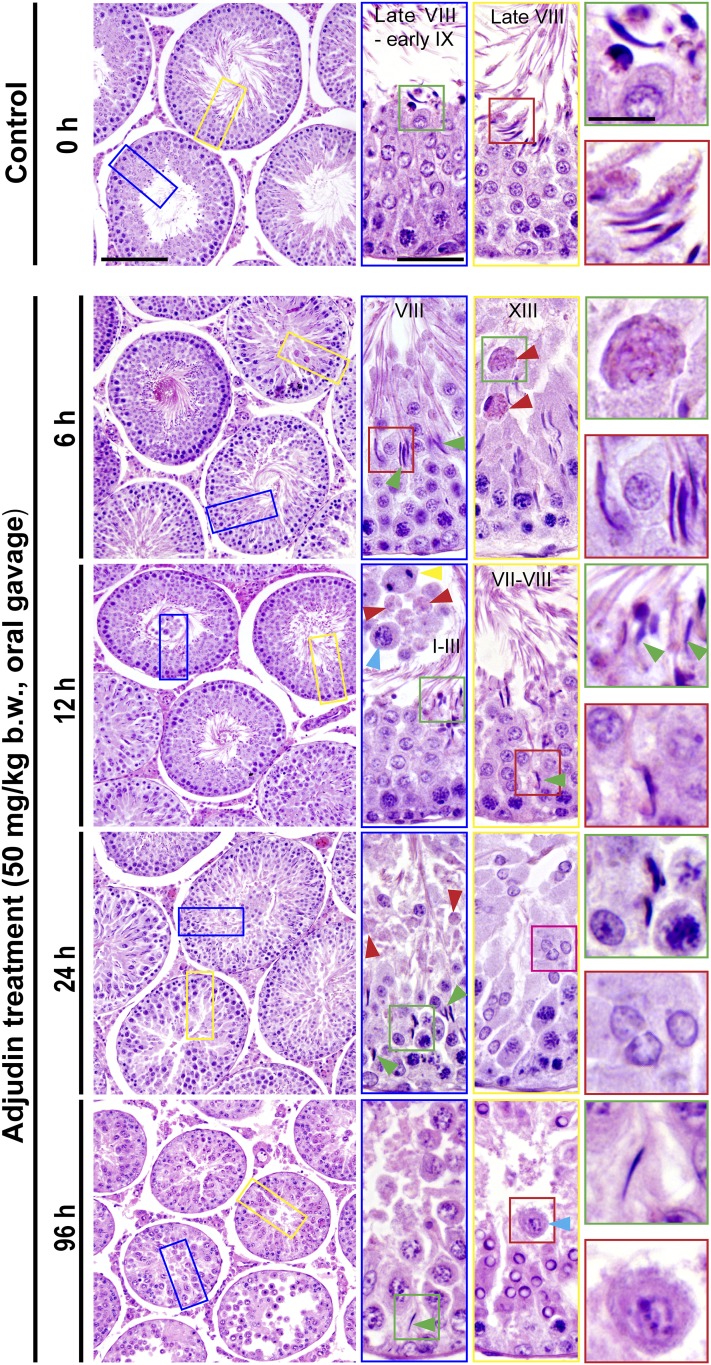
Adjudin treatment in adult rats rapidly induces defects in spermatogenesis prior to the occurrence of germ cell release from the seminiferous epithelium that mimics spermiation. Adult rats treated with a single dose of adjudin at 50 mg/kg b.w. by oral gavage at time 0 (controls; n = 5 rats). Thereafter, rats (n = 5) were terminated at specified time points at 6, 12, 24, and 96 hours vs 0 hours (control) for histological analysis using paraffin cross-sections of testes and H&E staining. By 6 and 12 hours, defects in spermatogenesis were noted in the testis. For instance, many elongated spermatids were trapped deep inside the epithelium (annotated by green arrowheads) when “unwanted spermiation” had taken place in nonstage VIII tubules but also stage VIII tubules; and phagosomes were found in a XIII tubule, which appeared to be derived from multinucleated round spermatids (annotated by red arrowheads; see also enlarged image in inset at 6 hours). In an apparently stage I to III tubule, a meiotic spermatocyte (annotated by a yellow arrowhead), phagosomes (annotated by red arrowheads), and a pachytene spermatocyte (annotated by a blue arrowhead) were detected in the tubule lumen when steps 15 and 16 spermatids (see green square box and the corresponding inset) were also depleting to the tubule lumen. By 24 hours, few elongating/elongated spermatids were found in the epithelium across the testis sections except those that were trapped deep inside the epithelium (annotated by green arrowheads and the corresponding boxed areas shown in insets), and both round spermatids, spermatocytes, and even some phagosomes were also emptied into the tubule lumen (see inset). At 96 hours (i.e., day 4), obvious defects in spermatogenesis were detected in the testis. For instance, virtually all elongated/elongated spermatids from 98% of tubules examined were not found in the seminiferous epithelium but depleted from the testis, yet some elongated spermatids remain trapped deep inside the epithelium, and this extensive germ cell loss also led to a reduction in tubule diameters. Scale bars = 180 µm in left panel at 0 hours, 70 µm in the blue boxed rectangle, and 30 µm in green boxed square, which apply to corresponding images in the same column or row.