Abstract
Rationale: Short-term follow-up in the Fluid and Catheter Treatment Trial (FACTT) suggested differential mortality by race with conservative fluid management, but no significant interaction.
Objective: In a post hoc analysis of FACTT including 1-year follow-up, we sought to estimate long-term mortality by race and test for an interaction between fluids and race.
Methods: We performed a post hoc analysis of FACTT and the Economic Analysis of Pulmonary Artery Catheters (EAPAC) study (which included 655 of the 1,000 FACTT patients with near-complete 1-year follow up). We fit a multistate Markov model to estimate 1-year mortality for all non-Hispanic black and white randomized FACTT subjects. The model estimated the distribution of time from randomization to hospital discharge or hospital death (available on all patients) and estimated the distribution of time from hospital discharge to death using data on patients after hospital discharge for patients in EAPAC. The 1-year mortality was found by combining these estimates.
Results: Non-Hispanic black (n = 217, 25%) or white identified subjects (n = 641, 75%) were included. There was a significant interaction between race and fluid treatment (P = 0.012). One-year mortality was lower for black subjects assigned to conservative fluids (38 vs. 54%; mean mortality difference, 16%; 95% confidence interval, 2–30%; P = 0.027 between conservative and liberal). Conversely, 1-year mortality for white subjects was 35% versus 30% for conservative versus liberal arms (mean mortality difference, −4.8%; 95% confidence interval, −13% to 3%; P = 0.23).
Conclusions: In our cohort, conservative fluid management may have improved 1-year mortality for non-Hispanic black patients with ARDS. However, we found no long-term benefit of conservative fluid management in white subjects.
Optimal fluid management for critically ill patients remains uncertain. Use of a conservative fluid strategy resulted in increased ventilator-free days and less organ dysfunction, but no difference in mortality for patients enrolled in the National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Network’s Fluid and Catheter Treatment Trial (FACTT) (1). The findings from this trial supported a number of earlier observational studies suggesting potential clinical benefits associated with conservative compared with liberal fluid management after reversal of shock (2–5).
It remains unclear whether the response to resuscitative fluids is homogenous across patient subgroups. Short-term follow-up suggested a mortality benefit for black patients treated with conservative fluid management, although there was not a significant interaction between fluid management and race (1). However, the short trial follow-up time may have masked a true interaction, and reevaluation of FACTT data with longer-term follow-up may better explain the effect of liberal fluid administration in different racial subgroups. Our aim, therefore, was to determine whether race modifies the effect of fluid administration strategy on 1-year mortality after ARDS. We hypothesized there would be a significant interaction between liberal fluid administration and black race, with black patients experiencing greater 1-year mortality.
Methods
Study Design and Participants
We performed a secondary data analysis of data obtained during the ARDS Network FACTT and Economic Analysis of Pulmonary Artery Catheters (EAPAC) study. Details for FACTT and EAPAC were published previously (1, 6, 7). Briefly, FACTT was a 2 × 2 factorial design trial evaluating two hemodynamic monitoring and fluid management strategies (1, 6). Participants received a central venous or pulmonary artery catheter (PAC) with administration of fluids (liberal fluid group) to maintain a prespecified venous pressure (central venous pressure 10–12 mm Hg or pulmonary artery occlusion pressure 14–18 mm Hg) or diuretic medication and/or fluid restriction (conservative fluid group) to maintain a central venous pressure below 4 mm Hg or pulmonary artery occlusion pressure below 8 mm Hg. FACTT subjects were followed until one of the following occurred: they returned home, they went off mechanical ventilation, or until day 60. EAPAC was a separately funded long-term follow-up study following subjects for 1 year to determine mortality, morbidity, and quality of life. Not all the original FACTT sites participated in EAPAC, and enrollment required separate consent for long-term follow-up. Death was adjudicated for EAPAC participants, using the fall 2006 National Death Index. The National Heart, Lung, and Blood Institute ARDS Network Steering Committee and institutional review boards at all participating sites approved both studies.
1-Year Mortality
We stratified FACTT subjects by treatment assignment and identified race into four categories: non-Hispanic black, liberal; non-Hispanic black, conservative; non-Hispanic white, liberal; and non-Hispanic white, conservative. Race was ascertained by the individual site study coordinators, reportedly by self-identification. We excluded Hispanic subjects from our analysis, given their small number and frequent mixed racial diversity. Because long-term follow-up was not available on all subjects, we used a multistate model to estimate the overall 1-year mortality for each of the four race/fluid groups, using the Markovian assumption that the probability of going to a future state depends only on the present state and not the history (8). Each subject could experience 3 possible states; the initial state was the index hospitalization, the intermediate state was going home after the index hospitalization, and the absorbing state was death. All subjects started in the index hospitalization. These patients could remain in this state, go home, or die; subjects discharged home after the index hospitalization could transition to the absorbing state of death. Once a subject left a certain state, it was not possible to return to that state.
We did not fit a model for rehospitalization, as the risk for subsequent hospitalization and death is included in the risk for death once a patient was discharged home after the index hospitalization, making this model unnecessary. We fit the multistate model within each race by fluid category to estimate overall 1-year mortality, and then tested the interaction between race and fluid treatment group. In essence, this approach imputes the 1-year mortality for subjects not followed after returning home or still requiring hospitalization at 60 days, using data from subjects in the same race treatment group who underwent 1-year follow-up. The model estimated the distribution of time from randomization to hospital discharge or hospital death (which was available on all patients) and estimated the distribution of time from hospital discharge to death, using data on patients after hospital discharge for patients in the follow-up study. The 1-year mortality was found by combining these estimates. Thus, some information was provided from all subjects. If a subject was known to survive 8 months, these data were used in the 12-month survival estimate. This method is an extension of the Kaplan-Meier estimator to multistate models. Our study has a special characteristic, in that a large portion of the patients were censored after they went home because their sites were not eligible for the study at the time, resulting in noninformative censoring. There were 217 black subjects: 57 were observed at 12 months, 81 were unobserved at 12 months because they were not eligible for EAPAC (33 died in hospital and 48 lacked follow up data), and 20 of the 136 black subjects enrolled in EAPAC were censored because of loss to follow-up. We performed a sensitivity analysis to determine the effect of missing data on our study findings (see Figures E1 and E2 in the online supplement). This sensitivity analysis showed there was very little chance that informative censoring in this group would have changed the inference.
We performed sensitivity analyses to determine whether the differential treatment effect on race was a result of variables associated with race that also affect mortality. We generated a propensity score for identification as black. The following predictors were included in the propensity score, defining the probability of identification as a black subject conditioning on each subject’s observed baseline characteristics: age, sex, Acute Physiology and Chronic Health Elements (APACHE) acute physiology score, APACHE chronic health score, partial pressure of oxygen (PaO2) to fractional inspired oxygen (FiO2) ratio, shock, central venous pressure (9), number of organ failures, prehospital days, and hospital site. FACTT employed an extensive quality control program that monitored target compliance to the protocol, on both a site and an individual level. Most sites were in the 85% compliance range, with only 4 sites reporting compliance between 70% and 80%. Participants were divided into four equal-size strata by estimated propensity score. Within each propensity score stratum, the distributions of observed baseline variables were similar for black- and white-identified subjects. A multistate model was fit for each stratum, and 1-year mortality was estimated. Estimated mortalities were averaged over the four strata with equal weights to obtain standardized 1-year mortality estimates for each of the race-by-fluid treatment categories. The multistate model was generated using the R MSTATE package. All other analyses were performed using SAS software, with a P value <0.05 considered statistically significant.
Results
Cohort Derivation
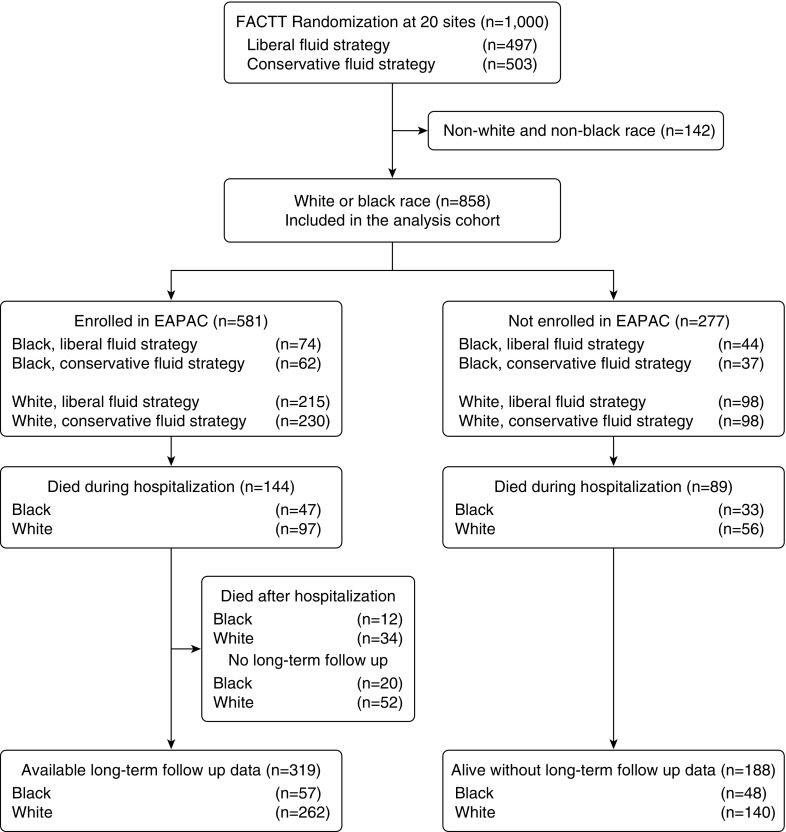
Coordinators identified 858 of the 1,000 subjects enrolled in FACTT (86%) as non-Hispanic white (n = 641, 75%) or black (n = 217, 25%) race (Figure 1). Treatment assignments for black subjects included 99 (46%) randomly assigned to a conservative and 118 (54%) to a liberal fluid management strategy (Table E1). Treatment assignments for white subjects included 313 (49%) randomly assigned to liberal and 328 (51%) to conservative fluid management. A total of 277 subjects were ineligible for EAPAC because of the lack of hospital participation in the study. The disposition of subjects is shown in Figure 1.
Figure 1.
Cohort derivation. FACTT = Fluids and Catheters Treatment Trial; EAPAC = Economic Analysis of Pulmonary Artery Catheters study.
Black subjects (Table 1) received care in a medical intensive care unit (ICU; 78% vs. 62%; P < 0.001) more often and were more likely to have a coexisting illness (47% vs. 30%; P = 0.003). Black subjects were more often diabetic (23% vs. 15%; P = 0.01) or had HIV infection or AIDS (14% vs. 4%; P < 0.01). APACHE III scores were higher, on average, for black subjects (mean 100 vs. 92; P < 0.01). Baseline tidal volumes were larger for white subjects compared with black subjects (428 vs. 413 mL; P = 0.03). There were no significant differences between groups stratified by fluid strategy (Table 2). The median length of stay in the ICU was 15 days (interquartile range, 7–18 days), with a median hospitalization of 20 days (interquartile range, 9–27 days). The average duration of follow-up was 161 days for the overall cohort. The average duration of follow-up for black subjects was 126 days; it was 173 days for white subjects.
Table 1.
Baseline characteristics and hospital outcomes stratified by race
| Variable | Non-Hispanic Black | Non-Hispanic White | All | P Value |
|---|---|---|---|---|
| N | 217 | 641 | 858 | |
| Age, mean (SD), yr | 48 (16) | 51 (16) | 50 (16) | 0.45 |
| Sex, female, n (%) | 98 (45) | 316 (49) | 414 (48) | 0.76 |
| Liberal fluid randomization group, n (%) | 118 (54) | 313 (49) | 431 (50) | 0.18 |
| Primary lung injury risk factor, n (%) | ||||
| Pneumonia | 115 (28) | 298 (72) | 413 (58) | <0.001 |
| Sepsis | 43 (20) | 146 (23) | 189 (22) | 0.34 |
| Aspiration | 30 (14) | 105 (16) | 135 (16) | 0.39 |
| Trauma | 15 (7) | 43 (7) | 58 (7) | 0.92 |
| Multiple transfusions | 1 (1) | 6 (1) | 7 (1) | 0.69 |
| Coexisting conditions, n (%) | ||||
| None | 114 (53) | 437 (70) | 551 (66) | <0.001 |
| Diabetes | 49 (23) | 92 (15) | 141 (17) | 0.12 |
| HIV infection or AIDS | 31 (14) | 25 (4) | 56 (7) | 0.10 |
| Cirrhosis | 8 (4) | 21 (3) | 29 (3) | 0.45 |
| Solid tumors | 4 (2) | 9 (1) | 13 (2) | 0.44 |
| Leukemia | 2 (1) | 17 (3) | 19 (2) | 0.13 |
| Lymphoma | 0 (0) | 11 (2) | 11 (1) | 0.08 |
| Immunosuppression | 19 (9) | 52 (8) | 71 (9) | 0.82 |
| APACHE III score, mean (SD) | 100 (32) | 92 (30) | 94 (31) | 0.002 |
| Medical ICU, n (%) | 169 (78) | 399 (62) | 568 (66) | <0.001 |
| Cardiorespiratory variables, mean (SD) | ||||
| Mean arterial pressure, mm/Hg | 77 (14) | 77 (14) | 77 (14) | 0.99 |
| Cardiac index, L/min/m2 | 4.4 (1.6) | 4.2 (1.4) | 4.2 (1.4) | 0.46 |
| Vasopressor use, n (%) | 86 (41) | 249 (40) | 335 (40) | 0.78 |
| Prerandomization fluid balance, L | 0.3 (0.8) | 0.3 (0.8) | 0.3 (0.8) | 0.36 |
| PaO2:FiO2 ratio | 127 (62) | 127 (57) | 127 (59) | 0.98 |
| Tidal volume (ml) | 413 (81) | 428 (86) | 424 (85) | 0.45 |
| Central venous pressure, cm H2O | 7 (9) | 8 (9) | 7 (9) | 0.47 |
| Central venous pressure ≤ 8 cm H2O, n (%) | 152 (72) | 459 (72) | 616 (72) | 1.00 |
| LOS, median (IQR) | ||||
| ICU LOS (d) | 16 (7–20) | 15 (7–18) | 15 (7–18) | 0.14 |
| Hospital LOS (d) | 20 (7–27) | 20 (9–27) | 20 (9–27) | 0.92 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Elements; ICU = intensive care unit; IQR = interquartile range; LOS = length of stay.
Table 2.
Fluid strategy assignment stratified by race
| Variable | Non-Hispanic Black |
P Value | Non-Hispanic White |
P Value | ||
|---|---|---|---|---|---|---|
| Liberal fluid strategy | Conservative fluid strategy | Liberal fluid strategy | Conservative fluid strategy | |||
| N | 118 | 99 | 313 | 328 | ||
| Age, mean (SD) | 48 (16) | 47 (17) | 0.82 | 50 (15) | 52 (16) | 0.11 |
| Sex, female, n (%) | 54 (55) | 44 (45) | 0.85 | 153 (48) | 163 (52) | 0.84 |
| Primary lung injury risk factor, n (%) | ||||||
| Pneumonia | 64 (54) | 51 (52) | 0.69 | 149 (48) | 149 (45) | 0.58 |
| Sepsis | 28 (24) | 15 (15) | 0.11 | 73 (23) | 73 (22) | 0.75 |
| Aspiration | 12 (16) | 14 (23) | 0.35 | 15 (13) | 15 (15) | 0.60 |
| Trauma | 7 (6) | 8 (8) | 0.53 | 21 (7) | 22 (7) | 0.99 |
| Multiple transfusions | 0 (0) | 1 (1) | 0.46 | 2 (1) | 4 (1) | 0.69 |
| Coexisting conditions, n (%) | ||||||
| None | 63 (54) | 51 (52) | 0.74 | 213 (71) | 224 (70) | 0.93 |
| Diabetes | 27 (23) | 22 (22) | 0.89 | 43 (14) | 49 (15) | 0.69 |
| HIV infection or AIDS | 19 (16) | 12 (12) | 0.39 | 13 (4) | 12 (4) | 0.73 |
| Cirrhosis | 4 (3) | 4 (4) | 0.81 | 12 (4) | 9 (3) | 0.43 |
| Solid tumors | 3 (3) | 1 (1) | 0.63 | 7 (2) | 2 (1) | 0.08 |
| Leukemia | 0 (0) | 2 (2) | 0.21 | 5 (2) | 12 (4) | 0.11 |
| Lymphoma | 0 (0) | 0 (0) | 5 (2) | 6 (2) | 0.99 | |
| Immunosuppression | 7 (6) | 12 (12) | 0.11 | 25 (8) | 27 (8) | 0.93 |
| APACHE III score, mean (SD) | 100 (32) | 98 (32) | 0.65 | 93 (29) | 91 (31) | 0.55 |
| Medical ICU, n (%) | 93 (79) | 76 (77) | 0.72 | 193 (63) | 206 (63) | 0.77 |
| Cardiorespiratory variables, mean (SD) | ||||||
| Mean arterial pressure, mm/Hg | 77 (13) | 77 (15) | 0.75 | 77 (15) | 77 (14) | 0.69 |
| Cardiac index, L/min/m2 | 5 (2) | 4 (1) | 0.46 | 4 (1) | 4 (1) | 0.97 |
| Central venous pressure, cm H2O | 8 (9) | 7 (9) | 0.47 | 8 (10) | 7 (9) | 0.47 |
| Central venous pressure ≤ 8 cm H2O, n (%) | 89 (75) | 68 (69) | 0.20 | 243 (74) | 216 (69) | 0.12 |
| Vasopressor use, n (%) | 48 (42) | 38 (39) | 0.62 | 126 (41) | 123 (38) | 0.39 |
| Prerandomization fluid balance, L | 0.3 (0.9) | 0.3 (0.8) | 0.92 | 0.4 (0.9) | 0.3 (0.8) | 0.63 |
| PaO2:FiO2 ratio | 130 (66) | 125 (58) | 0.51 | 125 (56) | 129 (58) | 0.41 |
| Tidal volume (ml) | 406 (77) | 421 (85) | 0.20 | 427 (86) | 430 (87) | 0.68 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Elements; ICU = intensive care unit.
1-Year Mortality
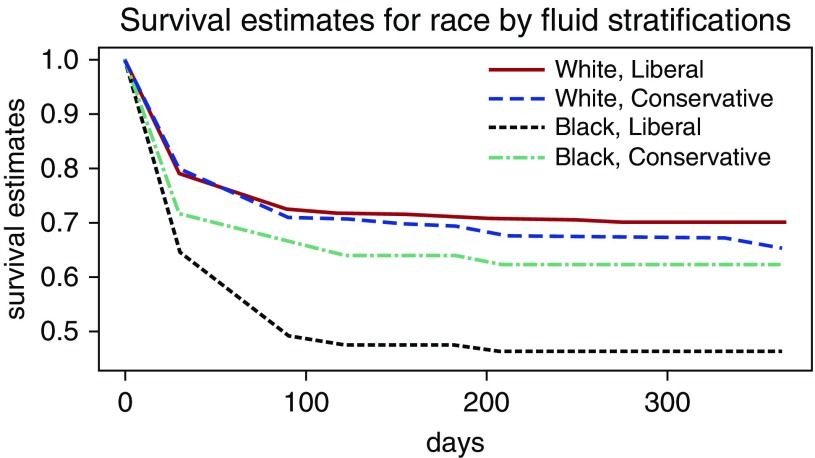
The 90-day hospital mortality rate for the entire study was 28%, whereas the 1-year mortality was estimated to be 35% (Table E2). The estimated 1-year mortality (Figure 2) was lower for subjects identified as black assigned to the conservative fluid group compared with those assigned to the liberal fluid group (38% vs. 54%), with a mean mortality difference (Table 3) of 16% (95% confidence interval [CI], 2–30%; P = 0.027). The estimated 1-year mortality was numerically greater for subjects identified as white assigned to the conservative fluid group compared with those assigned to the liberal fluid group (35% vs. 30%), but the mean mortality difference of −4.8% (95% CI, −13% to 3%; P = 0.23) was not significantly different for white subjects receiving the conservative fluid strategy. There was a significant interaction between identified race and fluid treatment group (P = 0.012).
Figure 2.
Cumulative mortality stratified by race and fluid strategy. The estimated 1-year mortality was greatest for black subjects assigned to the liberal fluid group. There was a significant interaction between identified race and fluid treatment group (P = 0.012).
Table 3.
1-year mortality difference between liberal and conservative fluid groups stratified by race
| Race | Conservative Fluid Strategy | Liberal Fluid Strategy | Difference | Difference Confidence Interval | P Value |
|---|---|---|---|---|---|
| Black | 38% ± 5.1% | 54% ± 5.1% | 16% ± 7.2% | 1.8 to 30% | 0.027 |
| White | 35% ± 2.9% | 30% ± 2.7% | −4.8% ± 4.0% | −13 to 3.0% | 0.23 |
| Interaction between race and fluid strategy | 21% ± 8.2% | 4.6 to 37% | 0.012 |
The estimated 1-year mortality changed minimally after propensity score adjustment (Table E3). After adjusting for the probability of identification as black, the estimated 1-year mortality for black subjects assigned to the liberal fluid group remained greater than for black subjects assigned to the conservative fluid group (47% vs. 33%). The estimated 1-year mortality for white-identified subjects assigned to the conservative fluid was greater after adjustment than the mortality for those assigned to the liberal fluid group (34% vs. 30%). The interaction between race and fluid administration remained statistically significant after propensity score adjustment (P = 0.044).
Discussion
Our primary aim in this study was to determine whether race modified the effect of fluid administration on mortality after ARDS. We originally hypothesized that longer-term follow-up would reveal a significant interaction of fluid management strategy and race, as the interaction term was of borderline significance in the original report (1). Our results suggest conservative fluid treatment may have reduced mortality in trial subjects with ARDS who identified as black. This finding confirms the original findings of FACTT and supports the hypothesis that liberal fluid administration may be particularly harmful in black patients. This finding conflicts with prior studies suggesting no racial differences in ARDS outcomes after adjustment for risk factors and systemic effects (10–12). Liberal fluid administration may be a potentially modifiable risk factor that may help explain observed racial differences in ARDS outcomes.
The primary benefit of the conservative fluid strategy in our study appeared driven by excess benefit in black subjects. A recent secondary analysis of FACTT data by Semler and colleagues (9) suggested that differences in baseline filling pressures contributed to excess volume administration in subjects with baseline central venous pressures less than or equal to 8 cm H2O. In multivariable modeling, each additional liter of fluid was associated with a 6% increase in mortality. Excess fluid administration, however, was associated with decreased mortality in the Caucasian subgroup (odds ratio, 0.51; 95% CI, 0.30–0.99; P = 0.045). We posit that the interaction between liberal fluids and race in black subjects may have contributed to the excess mortality observed with liberal fluids. In our cohort, propensity score adjustment using central venous pressure as a variable changed the mortality and interaction estimates minimally.
Defining the origin of racial disparities is challenging, and it is likely that a number of key factors contributed to the observed differences in our cohort. Before trying to study the effect of race, we had to have a plan to tackle two broad confounding issues. First, blacks and whites receive care disproportionately at different centers, so “race” may be a proxy for “center.” Second, “black” is a complex sociobiology construct such that identification as “black” may be a surrogate for variables associated with socioeconomic status, comorbid disease, severity of illness, or geographical location. Therefore, we adjusted for center and matched by the propensity to be black, where our propensity model attempted to capture many elements that may be collinear with self-identified race. However, we fully recognize that our dataset lacked important sociodemographic data to help explain the observed differences. Further, studies suggest there may be biologic differences in the response to fluid administration among differing racial groups. Studies of patients identified as black have shown less active renin-angiotensin systems (13) and lower bioavailability of nitric oxide when compared with patients identified as white, with resultant impairments in myocardial and vascular remodeling in response to oxidative stress (13). Polymorphisms in cytokine genotypes may be overrepresented in black subpopulations, with studies demonstrating up-regulation of interleukin 1β and interleukin 6 (14). Darc gene polymorphisms in subjects with ARDS who identified as black are shown to contribute to overexpression of interleukin 8, with subsequent associations with mortality and ventilator-free days (15). Finally, there are mixed data regarding long-term morbidity associated with ICU fluid administration. Positive fluid balance was significantly associated with inability to ambulate at hospital discharge in a cohort of sepsis survivors (16). Experimental data suggest excess tissue edema may contribute to local neuromuscular injury, increasing the likelihood for critical illness myopathy and neuropathy (17). Excess functional morbidity at ICU discharge in black survivors may contribute to longer-term mortality risk (18). Alternatively, the ARDS Cognitive Outcomes Study reported greater cognitive and psychiatric morbidity associated with conservative fluid management (19). It is possible that fluid effects are differential by organ system, but further studies are needed to fully understand the long-term effect of ICU volume administration.
Thus, we cannot ascribe causation to merely biologic or social factors but, rather, need an integrated biosocial approach to understanding ARDS disparities. One approach would consider volume overload as syndemic with ARDS severity, where the synergism between fluids and ARDS contributes to excess mortality in black patients with ARDS. Syndemics describe two or more diseases in a population with some level of deleterious biological or behavioral interface that exacerbates the negative health effects of any or all of the diseases involved (20, 21). Considering these factors syndemic highlights the multilevel factors and the potential between-level interactions that may lead to disparate critical care outcomes. The National Institutes of Health strongly suggest that all studies test for sex, race, and ethnicity interactions, and this suggestion is further strengthened by our findings (http://grants.nih.gov/grants/funding/women_min/guidelines_update.htm). Likewise, short-term mortality endpoints may not fully reflect the effect of an intervention on critical illness-related mortality, as effects may extend beyond the acute episode (22, 23). If critical illness extends beyond a standard follow-up period such as hospital discharge, short-term metrics may fail to capture the true effect of an intervention, as the mean effect may not be sustained or observed without longer-term monitoring.
There are some notable limitations to our study. As previously mentioned, the number of patients enrolled in the trial was low, with greater loss to follow-up and in-hospital mortality among black patients. Further, of the 1,000 original FACTT patients, 277 did not participate in the EAPAC follow-up study. We choose to use a Markov model to analyze these data with differential follow-up because there is substantial information on 1-year mortality in the data provided by these 277 patients who did not enroll in the follow-up study. We have assumed that the remaining patients also satisfied a missing-at-random assumption. Finally, although an analysis of treatment interaction with race was included in the original trial, the original trial was not powered to include longer-term follow-up. Since the early 1990s, the National Institutes of Health has required that the interactions of treatment with race and sex be tested in all clinical studies they fund. Thus, these analyses were preplanned. This does not completely solve the multiple comparison problem, as there are multiple race and sex hypotheses. Thus, the reported P value may not accurately reflect the probability of a type one error, and these data should be considered hypothesis-generating. However, as fluid strategies need to be determined for every patient, we believe it would be prudent to avoid high fluids for our black patients.
Conclusions
Our results suggest conservative fluid management may be particularly beneficial in patients who self-identified as black. However, we urge caution in overinterpretation, as our data did not contain important information regarding social determinants of health, including socioeconomic status, education level, or insurance status. Further studies are needed to understand why racial disparities in ICU mortality exist and to determine how fluid management strategies should be personalized for our ICU patients.
Supplementary Material
Footnotes
Supported by the National Heart, Lung, and Blood Institute’s Acute Respiratory Distress Syndrome (N01-HR56173) and Prevention and Early Treatment of Acute Lung Injury (PETAL) (U01 H123020) networks. The Economic Analysis of Pulmonary Artery Catheters study was funded by National Institutes of Health (NIH) grants R01-HS-11620 and N01-HR-46064. S.E.J. was supported by NIH T32 training grants HL007287-31 and 1 U54 GM104940 during the course of this research. D.S. and D.H. were supported by National Heart, Lung, and Blood Institute grants HHSN268200536179C and 5 U01HL123009.
Author Contributions: G.C., B.T.T., G.R.B., and D.C.A. contributed to study design and data collection of the primary Economic Analysis of Pulmonary Artery Catheters data; S.E.J., C.L.H., G.C., N.L.S., and D.C.A. contributed to design and data analysis for the current study; D.H., S.H., and D.S. conducted the data analysis for the current study; and all study authors contributed to manuscript preparation and revision and have reviewed the final manuscript and agree to its submission for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the ARDS Network Investigators
References
- 1.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 2.Rivers EP. Fluid-management strategies in acute lung injury: liberal, conservative, or both? N Engl J Med. 2006;354:2598–2600. doi: 10.1056/NEJMe068105. [DOI] [PubMed] [Google Scholar]
- 3.Schuller D, Mitchell JP, Calandrino FS, Schuster DP. Fluid balance during pulmonary edema: is fluid gain a marker or a cause of poor outcome? Chest. 1991;100:1068–1075. doi: 10.1378/chest.100.4.1068. [DOI] [PubMed] [Google Scholar]
- 4.Schuster DP. The case for and against fluid restriction and occlusion pressure reduction in adult respiratory distress syndrome. New Horiz. 1993;1:478–488. [PubMed] [Google Scholar]
- 5.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, et al. FEAST Trial Group. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 7.Clermont G, Kong L, Weissfeld LA, Lave JR, Rubenfeld GD, Roberts MS, Connors AF, Jr, Bernard GR, Thompson BT, Wheeler AP, et al. NHLBI ARDS Clinical Trials Network. The effect of pulmonary artery catheter use on costs and long-term outcomes of acute lung injury. PLoS One. 2011;6:e22512. doi: 10.1371/journal.pone.0022512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 9.Semler MW, Wheeler AP, Thompson BT, Bernard GR, Wiedemann HP, Rice TWNational Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome NetworkImpact of initial central venous pressure on outcomes of conservative versus liberal fluid management in acute respiratory distress syndrome Crit Care Med 201644782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34:2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JF, Zimmerman JE, Wagner DP, Hawkins M, Knaus WA. African-American and white patients admitted to the intensive care unit: is there a difference in therapy and outcome? Crit Care Med. 1995;23:626–636. doi: 10.1097/00003246-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R, Jr, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, et al. African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 14.Ness RB, Haggerty CL, Harger G, Ferrell R. Differential distribution of allelic variants in cytokine genes among African Americans and White Americans. Am J Epidemiol. 2004;160:1033–1038. doi: 10.1093/aje/kwh325. [DOI] [PubMed] [Google Scholar]
- 15.Kangelaris KN, Sapru A, Calfee CS, Liu KD, Pawlikowska L, Witte JS, Vittinghoff E, Zhuo H, Auerbach AD, Ziv E, et al. National Heart, Lung, and Blood Institute ARDS Network. The association between a Darc gene polymorphism and clinical outcomes in African American patients with acute lung injury. Chest. 2012;141:1160–1169. doi: 10.1378/chest.11-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell KH, Carlbom D, Caldwell E, Leary PJ, Himmelfarb J, Hough CL. Volume overload: prevalence, risk factors, and functional outcome in survivors of septic shock. Ann Am Thorac Soc. 2015;12:1837–1844. doi: 10.1513/AnnalsATS.201504-187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauptmann S, Klosterhalfen B, Weis J, Mittermayer C, Kirkpatrick CJ. Skeletal muscle oedema and muscle fibre necrosis during septic shock: observations with a porcine septic shock model. Virchows Arch. 1994;424:653–659. doi: 10.1007/BF00195781. [DOI] [PubMed] [Google Scholar]
- 18.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175:523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet. 2017;389:941–950. doi: 10.1016/S0140-6736(17)30003-X. [DOI] [PubMed] [Google Scholar]
- 21.Tsai AC, Mendenhall E, Trostle JA, Kawachi I. Co-occurring epidemics, syndemics, and population health. Lancet. 2017;389:978–982. doi: 10.1016/S0140-6736(17)30403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson ND, Scales DC, Pinto R, Wilcox ME, Cook DJ, Guyatt GH, Schünemann HJ, Marshall JC, Herridge MS, Meade MO Canadian Critical Care Trials Group. Integrating mortality and morbidity outcomes: using quality-adjusted life years in critical care trials. Am J Respir Crit Care Med. 2013;187:256–261. doi: 10.1164/rccm.201206-1057OC. [DOI] [PubMed] [Google Scholar]
- 23.Needham DM. Understanding and improving clinical trial outcome measures in acute respiratory failure. Am J Respir Crit Care Med. 2014;189:875–877. doi: 10.1164/rccm.201402-0362ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.