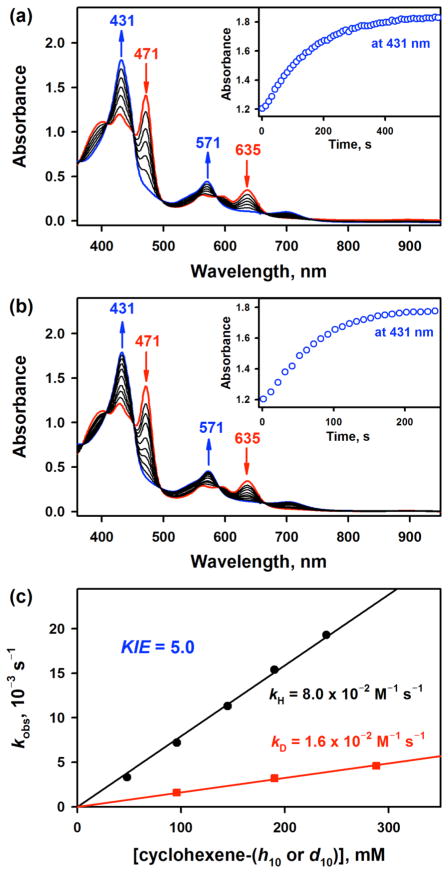
Figure 5.
(a) UV–vis spectral changes for the formation of 2 (blue line) and the disappearance of 3 (red line) upon addition of THF (0.12 M) to a CH3CN solution of [(TPFC)MnIII(OH)]− (3) (0.030 mM, red line) at –20 °C; 3 was prepared by adding TMAH (0.60 mM) to a CH3CN solution of [(TPFC)MnIII] (0.030 mM). Inset shows time course monitored at 431 nm due to 2. (b) UV–vis spectral changes for the formation of 2 (blue line) and the disappearance of 3 (red line) upon addition of cyclohexene (0.20 M) to a CH3CN solution of [(TPFC)MnIII(OH)]− (3) (0.030 mM, red line) at –20 °C; 3 was prepared by adding TMAH (0.60 mM) to a CH3CN solution of [(TPFC)MnIII] (0.030 mM). Inset shows time course monitored at 431 nm due to 2. (c) Plots of kobs against concentration of cyclohexene (black circles) and cyclohexene-d10 (red rectangles) to determine the second-order rate constants for the formation of 2 in the O2-activation by [(TPFC)MnIII] (0.030 mM) in the presence of TMAH (0.60 mM) in air-saturated CH3CN at –20 °C.