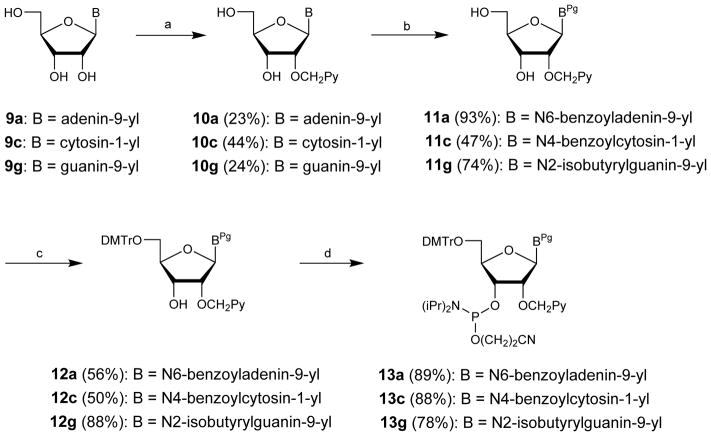
Scheme 4.
Synthetic routes to 2′-O-(pyren-1-yl)methyl-adenosine, -cytidine and -guanosine phosphoramidites:16,17 (a) PyCH2Cl, NaH, and DMF (9a and 9c) or DMSO (9g); (b) (i) TMSCl, pyridine, (ii) BzCl (10a and 10c) or isobutyrylchloride (10g), pyridine, (iii) aq. NH3; (c) DMTCl, pyridine; (d) (i-Pr2N)2PO(CH2)2CN, tetrazole, CH2Cl2 (12a and 12c) or NC(CH2)2OP(Cl)N(i-Pr)2, (i-Pr)2NEt, CH2Cl2 (12g). Pg = protecting groups.