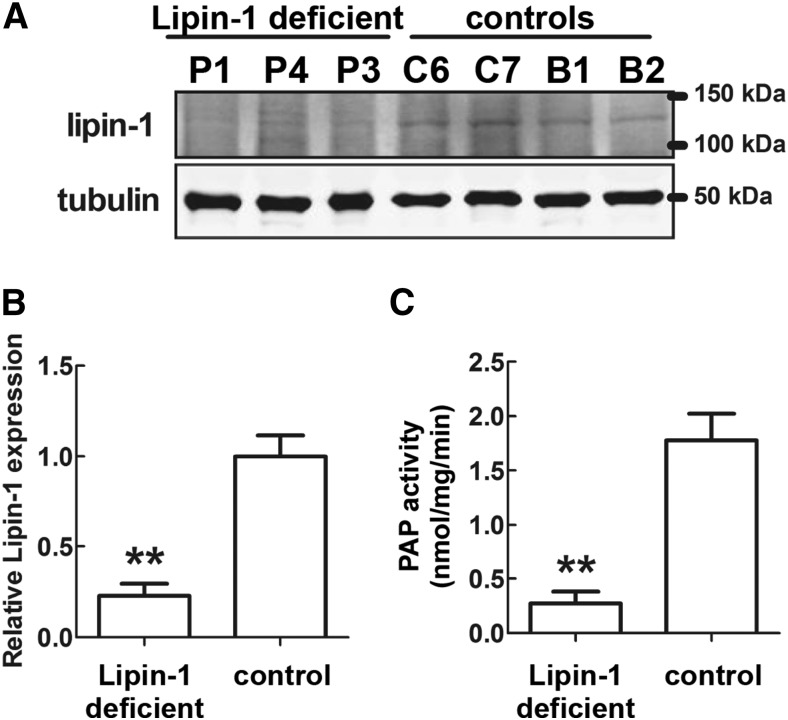
Fig. 4.
Lipin-1 protein levels and PAP activity in adipose tissue in patients carrying biallelic LPIN1 inactivating mutations. A: Western blot analysis of lipin-1 protein levels. Sixty micrograms of total protein extracts from adipose tissue biopsies from three patients and four normal controls were subjected to Western blot analysis, with the indicated specific antibodies. Two samples of breast adipose tissue (B1 and B2) were also used as controls due to the paucity of the biopsied subcutaneous WAT controls. P1 and P4: subcutaneous WAT from Patient 1 and Patient 4 (see Table 1), carrying the LPIN1 homozygous early stop mutations p.Arg388X/p.Arg388X; P3, subcutaneous WAT from Patient 3 (see Table 1) carrying the LPIN1 heterzygous mutations p.Asn417LysfsX22/p.Glu766_Ser838del (i.e., an early stop mutation and a C-terminal deletion); C6 and C7, subcutaneous WAT from normal individuals (Control 6 and Control 7, see Table 1); B1 and B2: control WAT from adult breasts obtained from two normal individuals, aged 53 and 56 years, subjected to reduction mastoplasty (see Methods). B: Densitometric analysis of the relative lipin-1 protein levels equalized for tubulin expression; protein levels were obtained from Western blots in A and shown as means ± SD of three (patients) or four (controls) independent determinations. C: PAP activity for the same samples shown in A. The values in B and C for the control’s adipose tissue showed that that both measures were tightly grouped for the subcutaneous WAT controls (C6 and C7) and for the breast WAT controls (B1 and B2) and did not depend on the anatomical location of the biopsied samples. In B and C, significant differences are indicated by **P < 0.01 (Student’s t-test).