Fig. 4. 3D-printed bacteria-functionalized structures with complex shapes for bioremediation and biomedical applications.
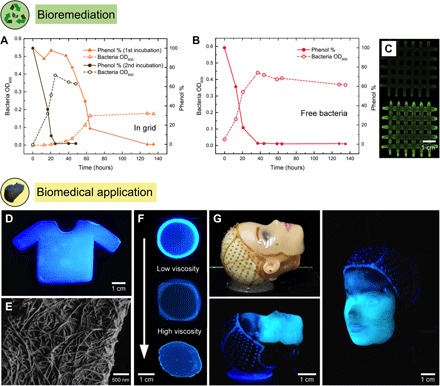
(A) A photo–cross-linked grid structure printed using a 4.5 wt % Flink-GMHA loaded with P. putida, a known phenol degrader, was incubated in an MM with phenol as the only carbon source. Phenol concentration and bacterial optical density (OD600) are shown as a function of time, as indicators of phenol degradation and bacterial growth. As a control, an equivalent bacteria concentration was incubated as a free-floating culture. (B) The preincubated grid was inoculated for a second time in a phenol containing MM. This time, phenol degradation happened as fast as with the free-floating control due to the higher bacterial concentration now present inside the grid. (C) Staining of bacterial DNA within the grid by ethidium bromide before (top) and after (bottom) incubation in the phenol-containing medium (343 nm). (D) In situ formation of bacterial cellulose by A. xylinum is used to generate a 3D-printed scaffold with a 4.5 wt % Flink in the shape of a T-shirt. Bacterial cellulose is visualized with a specific fluorescent dye at 365 nm. (E) Bacterial cellulose nanofibril network under SEM printed with a 3 wt % Flink. (F) Growth of bacterial cellulose depends on oxygen availability and the viscosity of the Flink. A dense cellulose network is only present in regions of high oxygen levels and low to medium viscosities. Images show, from top to bottom, circular prints using 3, 6, and 9 wt % Flinks. (G) A doll face was scanned, and a 4.5 wt % Flink containing A. xylinum was deposited onto the face using a custom-built 3D printer. In situ cellulose growth leads to the formation of a cellulose-reinforced hydrogel that, after removal of all biological residues, can serve as a skin transplant.
