Abstract
Background and objective
Standard nodal staging of lung cancer consists of positron emission tomography/computed tomography (PET/CT); followed by Endobronchial Ultrasound guided Trans bronchial Needle Aspiration (EBUS-TBNA) if PET/CT shows mediastinal lymphadenopathy. Sensitivity of EBUS-TBNA in patients with N0/N1 disease by PET/CT is unclear and largely based on retrospective studies. We assessed the sensitivity of EBUS-TBNA in this setting.
Methods
We enrolled patients with proven or suspected lung cancer staged as N0/N1 by PET/CT and without metastatic disease (M0), who underwent staging EBUS-TBNA. Primary outcome was sensitivity of EBUS-TBNA compared to a composite reference standard of surgical stage or EBUS-TBNA stage if EBUS demonstrated N2/N3 disease.
Results
Seventy-five patients were included in the analysis. Mean tumor size was 3.52 cm (±1.63). Fifteen out of 75 (20%) had N2 disease. EBUS-TBNA identified 6 while 9 were only identified at surgery. Sensitivity of EBUS-TBNA for N2 disease was 40% (95%CI: 16.3–67.7%).
Conclusion
A significant proportion of patients with N0/N1 disease by PET/CT had N2 disease (20%) and EBUS-TBNA identified a substantial fraction of these patients, thus improving diagnostic accuracy compared to PET/CT alone. Sensitivity of EBUS-TBNA however appears lower compared to historical data from patients with larger volume mediastinal disease. Therefore, strategies to improve EBUS-TBNA accuracy in this population should be further explored
Keywords: Bronchoscopy, Endosonography, Carcinoma, Non-Small-Cell Lung, Neoplasm Staging, Sensitivity and Specificity
Introduction
In the absence of distant metastases (M0), mediastinal nodal stage is a major determinant of treatment and prognosis1 in patients with non-small cell lung cancer. Standard nodal staging is initially performed non-invasively with positron emission tomography/computed tomography (PET/CT) followed by minimally invasive staging with Endobronchial Ultrasound-guided Transbronchial Needle Aspiration (EBUS-TBNA) when PET/CT is suggestive of mediastinal nodal involvement.
In patients without evidence of mediastinal nodal metastasis on PET/CT, the need for EBUS-TBNA becomes less clear. The most recent American College of Chest Physicians (ACCP) evidence-based guidelines2 recommend minimally invasive mediastinal staging with a needle technique in patients with a central tumor or nodal hilar disease (N1) on PET/CT. This is based on the increased prevalence of N2 disease in this group, but this is a grade 1C recommendation, i.e. based on low quality evidence. In patients with peripheral tumors and no evidence of mediastinal or hilar nodal disease on PET/CT, invasive staging is not recommended in the guidelines.
Concerns have been raised regarding these recommendations given that the prevalence of occult nodal metastasis in patients with N0 disease by PET/CT appears to be higher than previously reported, with recent studies showing values as high as 17–22% 3, 4. Furthermore, while mediastinal nodal staging is traditionally performed to rule out N2 disease and determine surgical eligibility, ruling out N1 disease is becoming increasingly relevant with the expanding use of stereotactic ablative radiotherapy (SABR).
Given the growing evidence that PET/CT may be insufficient for appropriate nodal staging5, 6, EBUS-TBNA may provide an attractive option to increase staging accuracy. Unfortunately the sensitivity of EBUS-TBNA in patients with a normal mediastinum (N0 or N1) by PET/CT is not clearly defined and is largely based on retrospective studies. We conducted a prospective study to determine the sensitivity and negative predictive value (NPV) of EBUS-TBNA for detecting N2 or N3 disease in this population. Additionally, we estimated the concordance of PET/CT stage with EBUS-TBNA stage, to assess the added value of EBUS/TBNA and describe the performance of EBUS to identify N1 disease in the subgroup of clinical N0 patients
Methods
Study design
This was a prospective cohort study of consecutive patients with early stage NSCLC undergoing EBUS-TBNA for mediastinal staging prior to definitive treatment. The protocol was approved by the Institutional Review Board (IRB protocol number 2007-0387). Written informed consent was obtained from all participants for both the clinical procedure and the study at the time of enrollment.
Patient selection
Subjects were eligible if they met all the following criteria: i) ≥18 years old ii)ECOG performance status of 0–2 iii) proven or suspected NSCLC iv) full radiographic work-up including contrast enhanced CT scan of the chest and a PET/CT within a month of the scheduled procedure and v) believed to be surgically resectable after initial clinical staging (N0 or N1 with no distant metastasis). If the patient was a survivor from a prior malignancy, inclusion also required a history of treatment with curative intent and no evidence of active or recurrent disease for the prior 5 years.
Patients who met any of the following criteria were not eligible: i) contraindication for EBUS-TBNA such as bleeding diathesis; ii) prior chemotherapy or radiation therapy for a thoracic malignancy; iii) separate tumors with the same histology (where primary versus metastatic disease was unclear) and iv) women who were pregnant or lactating.
Imaging tests
Lymph node enlargement by CT criteria was defined as size >1 cm on short axis. Standardized uptake values (SUV) ≥ 2.5 on PET/CT defined an FDG-avid lymph node. A tumor was defined as central if it was located within the inner third of the hemithorax and as peripheral if it was in the outer two thirds.
In the few cases where there was a discrepancy between the interventional pulmonologist and the radiologist regarding the radiologic stage by PET/CT, radiologic stage was assigned by two of the investigators who were unaware of the final pathological stage.
EBUS-TBNA
EBUS-TBNA was performed with a convex probe bronchoscope as previously described 7. Briefly, with the patient under general anesthesia, the bronchoscope was introduced and the intrathoracic lymph node stations were systematically examined to identify those meeting ultrasound criteria for sampling. These criteria included short axis diameter ≥ 0.5 cm and/or a combination of features associated with malignancy (e.g. sharp margins, heterogeneity, central necrosis sign, absence of a central hilar structure and rounded shape). Nodal sampling was performed beginning at the contralateral hilum and moving towards the N1 nodes. Using a 22-gauge needle, at least 3 passes were performed at each station sampled. Rapid on site cytological evaluation (ROSE) was available for all procedures. Adequate sample was defined by the presence of granulomas, malignancy or lymphocytes if the more cellular areas of direct smears contained at least 40 lymphocytes per high power field (i.e., 400× magnification). The lymph nodes stations were described according to the International Association for the Study of Lung Cancer classification.
Outcome measures
Our primary outcome was the sensitivity of EBUS-TBNA for N2 disease in patients with early stage NSCLC using a composite gold standard of either final pathologic stage or EBUS-TBNA stage if EBUS-TBNA showed N2 disease or higher. To assess the added value of EBUS-TBNA, we also estimated the concordance rate of the N descriptor between PET/CT and EBUS-TBNA.
Statistical analysis
Descriptive statistics such as the mean standard deviation (SD), median and range were used to summarize continuous variables. Frequencies and percentages were used to summarize categorical variables. Wilcoxon rank sum tests were used to compare continuous variables and Chi-squared tests were used to compare categorical variable. A p-value <0.05 was considered statistically significant. All analyses were performed using STATA software (version 13.0; SAS Institute, Cary, NC).
Results
Patients
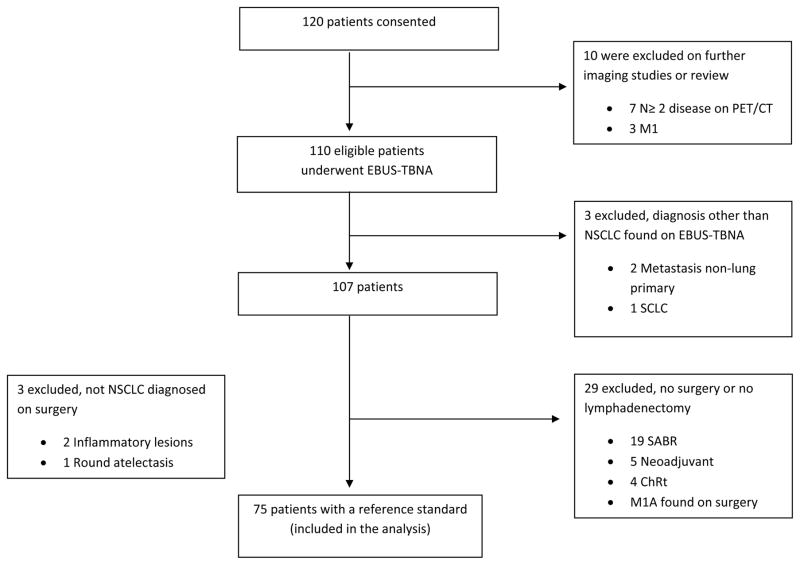
From April 2009 through February 2014, we consented a total of 120 participants. Eventually, 45 patients were excluded (Figure 1): 10 were excluded because they did not meet eligibility criteria based on further imaging studies or additional review of prior images demonstrating more advanced disease; 6 patients were found to have a diagnosis other than NSCLC, 3 by EBUS-TBNA and 3 at the time of surgery; and 29 patients did not undergo lymphadenectomy despite having N0 or N1 disease on EBUS-TBNA. A total of 75 patients had a reference standard allowing us to assess the primary outcome of EBUS TBNA sensitivity.
Figure 1.
Patient flow. SCLC, small cell lung cancer; SABR, stereotactic body radiation treatment; ChRT, chemoradiation treatment.
Among these 75 patients, the mean age was 66.3 years (±9.6), with 49% males. Mean size of the tumor on CT was 3.52 cm (±1.63), and 29% were centrally located. The majority of patients had clinical N0 disease (81%). Baseline characteristics are depicted in Table 1.
Table 1.
Baseline characteristics of included patients N=75 (%)
| Age, years | |
| Mean ± SD | 66.3 ± 9.6 |
|
| |
| Gender, n (%) | |
| Male, | 37 (49.3) |
|
| |
| Ethnicity, n (%) | |
| White | 61 (81.3) |
| Non-white | 14 (18.6) |
|
| |
| Smoking history, n (%) | |
| <20packs/year | 51 (68.0) |
| ≥20 packs/year | 24 (32.0) |
|
| |
| ECOG performance status, n (%) | |
| 0 | 49 (65.3) |
| 1 | 25 (33.3) |
| 2 | 1 (1.3) |
|
| |
| Tumor size on CT | |
| Mean ± SD | 3.5 ± 1.6 |
|
| |
| Localization, n (%) | |
| Central | 22 (29.0) |
| Peripheral | 53 (70.6) |
|
| |
| Histology, n (%) | |
| Adenocarcinoma | 51 (68.0) |
| Squamous cell carcinoma | 18 (24.0) |
| Other | 6 (6.8) |
|
| |
| FDG avidity, n (%) | |
| SUV ≥2.5 | 69 (92.0) |
| SUV<2.5 | 6 (8.0) |
|
| |
| Nodal stage prior to EBUS-TBNA, n (%) | |
| N0 | 61 (81.3) |
| N1 | 14 (18.6) |
EBUS-TBNA
The characteristics of the biopsied lymph nodes and sample adequacy among these are shown in Table 2. There were no complications from EBUS-TBNA.
Table 2.
Characteristics of EBUS-TBNA with lymph node station, size and adequacy
| Number of patients (Total of 75) | Lymph node size in cm, mean (range) | Adequate sampling, n (%) | |
|---|---|---|---|
| N3 | 41 | 0.69 (0.46–1.34) | 41 (100) |
| N2 paratracheal | 42 | 0.60 (0.29–0.97) | 42 (100) |
| N2 subcarinal | 56 | 0.81 (0.31–1.71) | 56 (100) |
| N1 | 51 | 0.73 (0.33–1.33) | 50 (98) |
| No lymph nodes sampled | 4 | <0.5 | Does not apply |
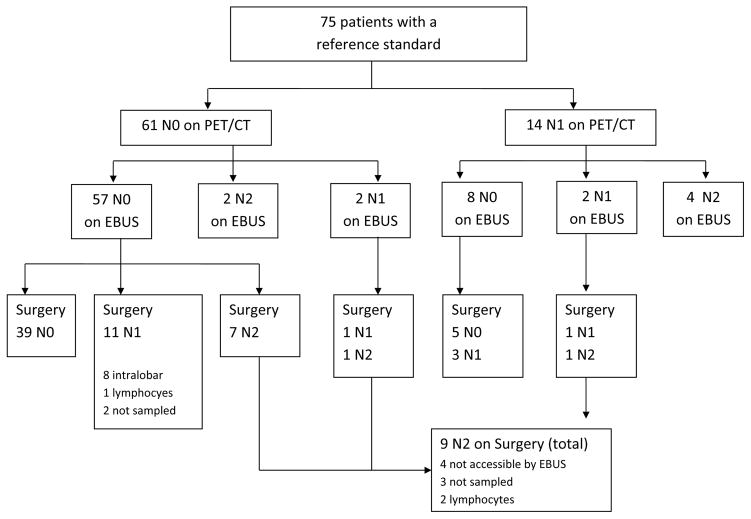
Overall, 23 out of 75 (30%) patients were eventually upstaged, with 15 out of 75 (20%) found to have N2 disease or higher (Figure 2). EBUS-TBNA identified 6 of these while the other 9 were only identified at surgery. Median time from EBUS-TBNA to surgery was 19 days (IQR: 11–28). Sensitivity of EBUS-TBNA for N2 disease among all evaluated patients compared to the reference standard was 40% (95% CI: 16.3–67.7%) with a negative predictive value of 89.9% (76.7–93.9). Among patients with EBUS accessible nodal disease, defined as stations 1, 2, 4, 3P, 7, 10 and 11, the sensitivity was 54.5% (95% CI: 23.4–83.2), and the negative predictive value was 92.3% (95% CI:84–98). Test characteristics are shown in Table 3.
Figure 2.
Patient flowchart with nodal stage by PET/CT, EBUS-TBNA and surgery.
Table 3.
Diagnostic performance of EBUS-TBNA
| All lymph node stations, % (95% CI) | EBUS-accessible lymph nodes only, % (95%CI) | |
|---|---|---|
| Sensitivity | 40.0 (16.3–67.7) | 54.5 (23.4–83.2) |
| Specificity | 100 (94.0–100) | 100 (94.0–100) |
| Negative predictive value | 89.9 (76.7–93.9) | 92.3 (84.0–98.0) |
| Negative Likelihood ratio | 0.60 (0.40–0.90) | 0.45 (0.24–0.87) |
The proportion of patients concordantly staged as less than N2 with both PET/CT and EBUS-TBNA was 92% (95% CI: 83.4–97.0%). After performing an EBUS-TBNA, the probability of having occult N2 disease was reduced from 20% (15/75) to 12% (9/75). In other words, the number of patients needing to undergo an EBUS-TBNA in order to avoid one case of N2 disease or higher being discovered at surgery would be 12.5.
The characteristics of patients with occult N2 disease are shown in Table 4. On bivariate analyses, there were no statistically significant differences between patients with or without occult lymph node disease regarding age, tumor size, and histology or tumor location.
Table 4.
Characteristics of the lymph nodes representing occult N2 disease
| Patient | N stage on PET/CT | Nodal station | Size of the LN (cm) | Cytology | Tumor | ||
|---|---|---|---|---|---|---|---|
| CT | EBUS | Lobe/situation | Size in cm | ||||
| N2 disease found on surgery (False negative of EBUS) | |||||||
|
| |||||||
| 1 | N0 | 5 | 0.76 | NA | NA | LUL/central | 0.9 |
| 2 | N0 | 3A | 0.61 | NA | NA | RLL/central | 1.8 |
| 9 | 0.96 | NA | NA | ||||
| 3 | N0 | 5 | 0.61 | NA | NA | LUL/central | 3.8 |
| 4L | 0.96 | Not seen | Not sampled | ||||
| 4 | N0 | 5 | 0.86 | NA | NA | LUL/central | 3.7 |
| 5 | N0 | 5 | 0.48 | NA | NA | LLL/peripheral | 2.5 |
| 6 | 0.55 | NA | NA | ||||
| 6 | N0 | 7 | 0.3 | 0.38 | Not sampled | LLL/peripheral | 2.3 |
| 7 | N0 | 7 | 0.59 | 0.67 | Lymphocytes | RUL/peripheral | 1.7 |
| 8 | N0 | 4R | 0.43 | 0.42 | Not sampled | RLL/central | 4.5 |
| 9 | N1 | 7 | 0.9 | 1.30 | Lymphocytes | RLL/peripheral | 6.0 |
|
| |||||||
| N2 disease found on EBUS | |||||||
|
| |||||||
| 10 | N0 | 4L | 0.82 | 0.66 | Malignancy | LLL/peripheral | 6.8 |
| 11 | N1 | 4L | 0.3 | 0.3 | Malignancy | LUL/central | 3.5 |
| 12 | N1 | 4L | 0.65 | 0.65 | Malignancy | LUL/peripheral | 3.0 |
| 13 | N1 | 4R | 0.65 | 0.6 | Malignancy | RUL/peripheral | 3.6 |
| 14 | N0 | 4L | 0.74 | 0.54 | Malignancy | LUL/peripheral | 2.8 |
| 7 | 0.95 | 0.99 | Malignancy | ||||
| 15 | N1 | 4R | 0.54 | 0.84 | Malignancy | RUL/peripheral | 3.8 |
Subgroup of clinical N0 patients
Among patients who had clinical N0 disease (N=61), 22 (36%) were eventually found to have nodal metastasis, 12 were upstaged to N1 (19%), and 10 were upstaged to N2 disease (16%). (Figure 2). Of the 10 patients upstaged to N2, 5 had peripheral tumors (Table 4).
Of the 12 patients upstaged to N1, only one patient was identified by EBUS-TBNA. Of the 11 patients with false negatives, 8 were from intraparenchymal lymphnodes not accessible by EBUS-TBNA, one was sampled and adequate lymphoid tissue was obtained, and two were not sampled because they did not meet size or morphological criteria for sampling. Of the 10 patients upstaged to N2 disease or higher, only two were identified by EBUS. Of the 8 patients with false negatives, 4 were located in stations not accessible by EBUS-TBNA, 3 were not sampled because they did not meet size or morphological criteria, and 1 was sampled and adequate lymphoid tissue was obtained.
Subgroup of clinical N1 patients
Fourteen patients had clinical N1 disease. Five of the 14 (36%) were eventually upstaged to N2 disease, 4 were confirmed as N1, and the remaining 5 were down staged to N0.
Of the 5 patients upstaged from clinical N1 to N2, 4 (80%) were identified by EBUS-TBNA. The only false negative was at a subcarinal lymph node where adequate lymphocytes were obtained. Of the 4 patients eventually confirmed as N1 disease, 3 had been wrongly down staged by EBUS-TBNA. Two out of these 3 patients were located in stations not accessible by EBUS (intralobar); and on the one remaining patient, adequate lymphocytes were obtained.
The sensitivity of EBUS-TBNA for N2 disease was not statistically different in the clinical N0 group (20%, 95% CI 2.52–55.6) compared to the clinical N1 group (80%, 95% CI 28.4–99.5).
Discussion
In this prospective study of patients with NSCLC and no evidence of mediastinal nodal metastasis on PET/CT we found that EBUS-TBNA has a sensitivity of 40% in the identification of N2 disease. While the sensitivity is lower than previously reported, these findings also suggest that EBUS-TBNA can improve the preoperative staging of these patients compared with standard radiographic staging with PET/CT, given the 92% concordance rate.
A surprising 20% of patients with N0 or N1 disease by clinical radiographic criteria were found to have occult N2 disease. As expected, patients with clinical N1disease had a higher rate of occult N2 disease (36%), and EBUS-TBNA detected 80%of them. Occult N2 disease was present not only among those with central tumors or N1disease, but also in 5 out of 61 (8%) patients with N0 disease and peripheral tumors, a group where current ACCP guidelines do not recommend any form of preoperative lymph node sampling. These findings may have important implications in clinical management particularly if SABR is being considered.
Additionally, EBUS-TBNA was useful in identifying diagnosis other than NSCLC that did not require surgery as the first choice of treatment (3 out of 110 eligible patients). These patients in addition to those found to have N2 disease (6 patients) compose a group of patients in which an unnecessary thoracotomy was avoided.
The sensitivity of EBUS-TBNA in this study is lower than described in the setting of larger volume mediastinal disease2 and consistent with more recent studies in similar populations that reported sensitivities around 35% 3, 8. The lower sensitivity is at least partially explained by lymph nodes in locations not accessible to EBUS-TBNA and possibly the presence of small volume metastasis in lymph nodes without ultrasound features of malignancy.
The generalizability of our findings may be limited by the fact that the procedures were all performed under general anesthesia, with ROSE availability and in a high volume center. Also, a substantial number of enrolled patients eventually elected to undergo treatments other than surgery and as such did not have a surgical confirmation. Since these patients were excluded from the primary outcome analysis, this may have introduced bias into our sensitivity estimate.
The strengths of this study include the prospective collection of data and that the imaging was always obtained within a month of undergoing an EBUS-TBNA, and surgery was performed generally within 30 days of the EBUS-TBNA, reducing the time window for interval nodal upstaging.
Although initial studies on the sensitivity of EBUS in this population were higher 9, this study adds to the growing body of evidence demonstrating that EBUS-TBNA may have only a moderate sensitivity, in this setting and raises the possibility that the diagnostic utility of EBUS may be different in the clinical N0 and N1 groups. A recent report has even suggested that among patients with cN1 disease and negative N2 nodes on EBUS-TBNA, a mediastinoscopy may also be considered to rule out N2 disease8. Since some of the false negative results in this particular study were from lymph nodes that did not meet current size thresholds for biopsy, our results suggest possible alterations in the current sampling protocols in this patient population including modifying the currently used size threshold for biopsy, systematic sampling of stations 4 and 7 regardless of size, sampling multiple lymph nodes at each station, lymph node elastography, combined EBUS and esophageal ultrasound or perhaps even incorporating the use of genomic, proteomic or other more sensitive biomarkers for biopsy sample analysis. Whether a more thorough sampling of the mediastinal lymph nodes in this setting, as suggested by some investigators10, would improve the accuracy of staging with TBNA is unknown but should be the subject of future studies.
The combined EBUS-EUS strategy for nodal staging was examined in a recent meta-analysis, showing a modest increase in sensitivity 11. These findings were largely based on a population with standard guideline recommended indications for invasive mediastinal staging based on either abnormal lymph nodes or central tumors. In our study, only one of the patients eventually diagnosed with N2 disease involved a lymph node accessible exclusively by EUS (station 9) but not EBUS-TBNA.
The best staging strategy in terms of accuracy, cost-effectiveness, and safety in the setting of negative results on PET/CT is currently unclear and should also be the subject of further investigation. Given the limitations of various testing modalities, it is unlikely to be a single definitive test but rather a composite estimate based on an assessment of the pretest probability, minimally invasive sampling and the possible consequence of missed nodal disease.
In conclusion, the prevalence of occult mediastinal nodal metastasis can be as high as 20% in patients with a radiographically normal mediastinum by PET/CT. EBUS-TBNA may be useful in identifying up to 40% of these patients and should be considered in this setting particularly in patients with clinical N1 disease.
Summary at a Glance.
We assessed the sensitivity of EBUS-TBNA in patients with lung cancer staged as N0/N1 by PET/CT. EBUS-TBNA improved the pretreatment staging of these patients compared with PET/CT, however the sensitivity for N2 disease is only 40%, lower than sensitivities previously reported for N2/N3 patients.
Acknowledgments
This research is supported in part by the National Institutes of Health Grant CA016672
References
- 1.Rusch VW, Crowley J, Giroux DJ, Goldstraw P, Im JG, Tsuboi M, Tsuchiya R, Vansteenkiste J. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:603–12. doi: 10.1097/JTO.0b013e31807ec803. [DOI] [PubMed] [Google Scholar]
- 2.Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, Harris LJ, Detterbeck FC. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e211S–50S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 3.Ong P, Grosu H, Eapen GA, Rodriguez M, Lazarus D, Ost D, Jimenez CA, Morice R, Bandi V, Tamara L, Cornwell L, Green L, Zhu A, Casal RF. Endobronchial ultrasound-guided trans bronchial needle aspiration for systematic nodal staging of lung cancer in patients with N0 disease by computed tomography and integrated positron emission tomography-computed tomography. Ann Am Thorac Soc. 2015;12:415–9. doi: 10.1513/AnnalsATS.201409-429OC. [DOI] [PubMed] [Google Scholar]
- 4.Shingyoji M, Nakajima T, Yoshino M, Yoshida Y, Ashinuma H, Itakura M, Tatsumi K, Iizasa T. Endobronchial ultrasonography for positron emission tomography and computed tomography-negative lymph node staging in non-small cell lung cancer. Ann Thorac Surg. 2014;98:1762–7. doi: 10.1016/j.athoracsur.2014.05.078. [DOI] [PubMed] [Google Scholar]
- 5.Shin KM, Lee KS, Shim YM, Kim J, Kim BT, Kwon OJ, Park K. FDG PET/CT and mediastinal nodal metastasis detection in stage T1 non-small cell lung cancer: prognostic implications. Korean J Radiol. 2008;9:481–9. doi: 10.3348/kjr.2008.9.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bille A, Pelosi E, Skanjeti A, Arena V, Errico L, Borasio P, Mancini M, Ardissone F. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothorac Surg. 2009;36:440–5. doi: 10.1016/j.ejcts.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Casal RF, Lazarus DR, Kuhl K, Nogueras-González G, Perusich S, Green LK, Ost DE, Sarkiss M, Jimenez CA, Eapen GA, Morice RC, Cornwell L, Austria S, Sharafkanneh A, Rumbaut RE, Grosu H, Kheradmand F. Randomized trial of endobronchial ultrasound-guided trans bronchial needle aspiration under general anesthesia versus moderate sedation. Am J Respir Crit Care Med. 2015;191:796–803. doi: 10.1164/rccm.201409-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dooms C, Tournoy KG, Schuurbiers O, Decaluwe H, De Ryck F, Verhagen A, Beelen R, van der Heijden E, De Leyn P. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: a prospective multicenter study. Chest. 2015 Jan;147(1):209–15. doi: 10.1378/chest.14-0534. [DOI] [PubMed] [Google Scholar]
- 9.Herth FJ, Eberhardt R, Krasnik M, Ernst A. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum inpatients with lung cancer. Chest. 2008 Apr;133(4):887–91. doi: 10.1378/chest.07-2535. [DOI] [PubMed] [Google Scholar]
- 10.Detterbeck F, Puchalski J, Rubinowitz A, Cheng D. Classification of the thoroughness of mediastinal staging of lung cancer. Chest. 2010;137:436–42. doi: 10.1378/chest.09-1378. [DOI] [PubMed] [Google Scholar]
- 11.Korevaar DA, Crombag LM, Cohen JF, Spijker R, Bossuyt PM, Annema JT. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med. 2016 Dec;4(12):960–968. doi: 10.1016/S2213-2600(16)30317-4. [DOI] [PubMed] [Google Scholar]