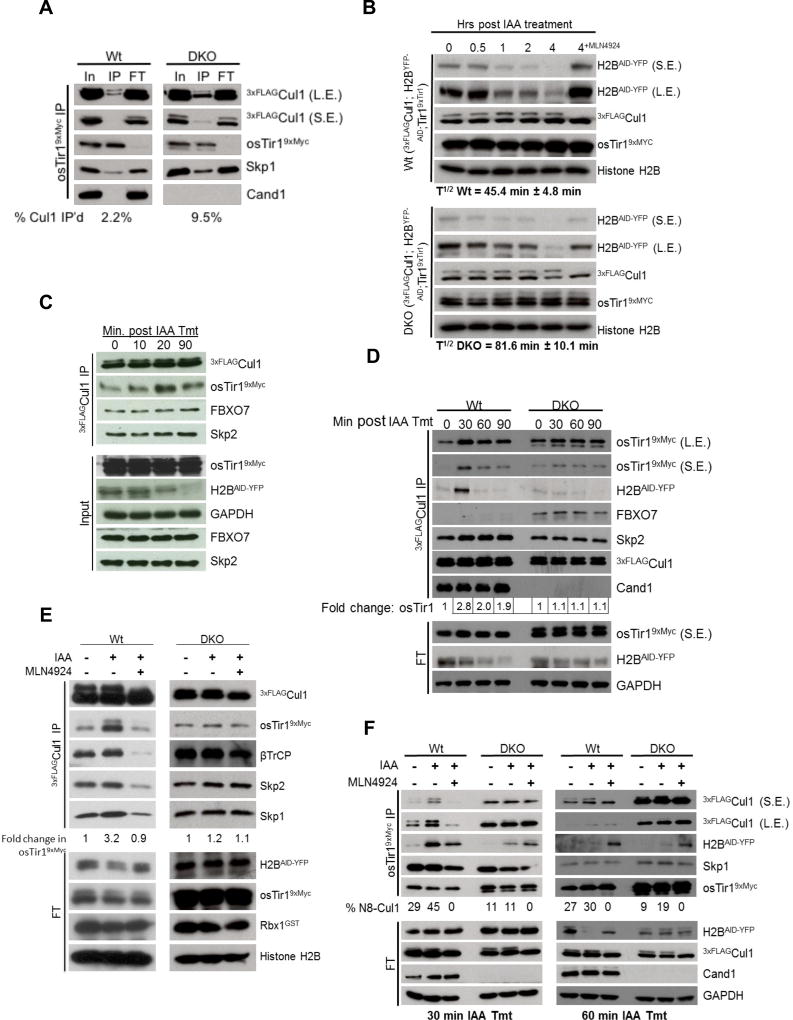
Fig. 7. Substrate drives SCF complex assembly in a Cand1/2- and neddylation-dependent manner.
(A) Steady state assembly of osTir19xMyc with 3xFLAGCul1 in Wt and DKO cells in the absence of auxin. Wt and DKO HEK2933xFLAG-Cul1 cells stably expressing osTir19xMyc and Tet-inducible H2BAID-YFP were lysed in the presence of rCul1GSTRbx1 and subjected to IP with anti-Myc. The bound (IP) and unbound (FT) fractions were Western blotted with the indicated antibodies. (B) Degradation of H2B AID-YFP substrate is hindered in the absence of Cand1/2. The cell lines from (A) were treated with tetracycline for 24 hrs to activate H2BAID-YFP expression, treated with 500 µM auxin, and at the indicated time points after auxin addition samples were lysed and analyzed by Western blot. The half-life of H2BAID-YFP was calculated from 2 biological replicates. (C) Auxin-induced osTir1 AID-YFP assembly with Cul1 is rapid. Wt cells from (A) were treated with 500 µM auxin for the indicated time, lysed in the presence of rCul1GSTRbx1, and subjected to IP followed by Western blot. (D) Auxin-driven SCFTir1 assembly peaks in 30’ and depends on Cand1/2. Cells from (A) were treated as described in (C) for the indicated amount of time. Fold change in osTir19xMyc association with 3xFLAGCul1 is displayed below. (E) Inhibition of neddylation blocks substrate driven SCF complex assembly. Same as (D) except cells were treated ± MLN4924 and auxin, as indicated, for 30’ prior to cell lysis. (F) Substrate increases the association of neddylated Cul1 interacting with osTir19xMyc. Cells were treated and processed as described above except that auxin treatment was for 30’ or 60’ and IP was for osTir19xMyc. Percent neddylated Cul1 (N8-Cul1) is indicated. S.E., L.E.: short and long exposures.