Abstract
Background and Objectives
This trial evaluated the safety and efficacy of the Genoss drug-eluting coronary stent.
Methods
This study was a prospective, multicenter, randomized trial with a 1:1 ratio of Genoss drug-eluting stent (DES)™ and Promus Element™. Inclusion criteria were the presence of stable angina, unstable angina, or silent ischemia. Angiographic inclusion criteria were de novo coronary stenotic lesion with diameter stenosis >50%, reference vessel diameter of 2.5–4.0 mm, and lesion length ≤40 mm. The primary endpoint was in-stent late lumen loss at 9-month quantitative coronary angiography follow-up. Secondary endpoints were in-segment late lumen loss, binary restenosis rate, death, myocardial infarction (MI), target lesion revascularization (TLR), target vessel revascularization (TVR), and stent thrombosis during 9 months of follow-up.
Results
We enrolled 38 patients for the Genoss DES™ group and 39 patients for the Promus Element™ group. In-stent late lumen loss at 9 months was not significantly different between the 2 groups (0.11±0.25 vs. 0.16±0.43 mm, p=0.567). There was no MI or stent thrombosis in either group. The rates of death (2.6% vs. 0%, p=0.494), TLR (2.6% vs. 2.6%, p=1.000), and TVR (7.9% vs. 2.6%, p=0.358) at 9 months were not significantly different.
Conclusion
This first-in-patient study of the Genoss DES™ stent showed excellent angiographic outcomes for in-stent late lumen loss and major adverse cardiac events over a 9-month follow-up.
Keywords: Drug-eluting stents, Coronary artery disease, Sirolimus
INTRODUCTION
Percutaneous coronary intervention (PCI) using a drug-eluting stent (DES) is a standard treatment for significant coronary artery occlusive disease. First-generation DESs have shown significantly lower restenosis rates compared to bare metal stents.1),2),3),4) Although DES reduced the rate of restenosis and target lesion revascularization (TLR), the risk of stent thrombosis increased.5),6),7)
Stent thrombosis is a clinically serious complication because of its high mortality rate. It is related to early discontinuation of antiplatelet agents, resistance to antiplatelet agents, delayed endothelization, hypersensitivity reaction to polymers, and several clinical or procedural factors.6),7),8) As one possible cause of stent thrombosis is a hypersensitivity reaction to a durable polymer, the Nobori™ stent (Terumo Corporation, Tokyo, Japan) and BioMatrix™ (Biosensors International, Singapore) used a bioresorbable polymer that did not remain in the stent after a certain time period and showed good clinical outcomes compared to a durable polymer DES.9),10)
The Genoss DES™ stent (Genoss Company Limited, Suwon, Korea) is the first sirolimus-eluting stent with biodegradable polymers made in Korea. The objectives of this first-in-patient prospective randomized study were to evaluate the efficacy and safety of the Genoss DES™ stent in comparison to the Promus Element™ (Boston Scientific, Natick, MA, USA).
METHODS
Patient population
This study was a prospective, multicenter, single blind (patient blind), randomized study with 1:1 ratio of patients using the Genoss DES™ stent and Promus Element™, conducted from March 2013 to April 2015. The study enrolled 80 patients at 4 Korean centers. Inclusion criteria were the presence of stable angina, unstable angina, or silent ischemia in patients between 20 and 80 years of age. Angiographic inclusion criteria were de novo coronary stenotic lesion with diameter stenosis (DS) >50%, reference vessel diameter (RVD) of 2.5 to 4.0 mm, and a lesion length ≤40 mm. Exclusion criteria were the presence of acute myocardial infarction (MI), cardiogenic shock, low ejection fraction (<40%), contraindication to antiplatelet agents, and chronic total occlusion lesion, restenosis, left main disease, and stenosis of the graft vessel. The protocol was approved by the Institutional Review Board of the Ajou University Hospital, Yonsei University Wonju Christian Hospital, Seoul National University Hospital, and Seoul St. Mary's Hospital, and all patients provided written informed consent.
The Genoss DES™ sirolimus-eluting stent
The Genoss DES™ is a sirolimus-eluting cobalt-chromium coronary stent system for the treatment of coronary artery diseases. The stent platform features open-cell 2 links with a uniform architecture that permits a high level of flexibility with good radial force, shortening, and conformability. Two kinds of strut thickness (70 and 78 µm) are adopted, depending on the stent diameter (2.25–2.50 and 2.75–4.00 mm, respectively). To achieve favorable clinical outcomes, the initial burst release of drug from DES is needed to suppress vascular injury and inflammation induced by catheter manipulation and stent implantation. Accordingly, the Genoss DES™ is designed to release approximately 70% of its initial drug payload (1.15 µg/mm2) within the first 30 days following implantation. To achieve the desired drug release profile and minimize the amount of polymer, a 4 µm ultra-thin coating is applied to only the abluminal side of the stent. This bioresorbable coating consists of a proprietary blend of poly-DL-lactide-co-glycolide (PLGA) and poly-DL-lactide (PLA), which are almost fully resorbed within 9 months.
Study procedure
PCI was performed using standard interventional techniques. Intravenous heparin was administered to maintain an activated clotting time of more than 250 seconds, and antiplatelet therapy was used before the procedure with aspirin (100–325 mg) and clopidogrel (loading dose of 300–600 mg) or ticlopidine (500 mg/day). Dual antiplatelet therapy was maintained during the 9 months of follow-up. Glycoprotein IIb/IIIa receptor antagonists were used at the operator's discretion. After predilation, Genoss DES™, or Promus Element™ was implanted under rated burst pressure, and post-dilatation was performed if needed. This study permitted 2 stents to overlap in cases of long lesions.
Follow-up
Clinical follow-up was performed at the time of discharge, and following a period of 1, 5, and 9 months. All patients were planned to undergo a follow-up angiography and intravascular ultrasound (IVUS) at 9±1 months.
Quantitative coronary angiography and intravascular ultrasound
Quantitative coronary angiography (QCA) analyses were performed by 2 independent observers blinded to treatment group using the Cardiovascular Angiography Analysis System II (CAAS II; Pie Medical, Maastricht, Netherlands). The percent DS, minimal luminal diameter, RVD, and lesion length were measured and calculated before the intervention, at the end of the procedure, and at the 9-month follow-up.
IVUS was performed at the end of the index procedure and at follow-up, and analyzed by 2 independent observers blinded to clinical and procedural information according to the American College of Cardiology clinical expert consensus document on standards for acquisition, measurement, and reporting of IVUS studies.11) All IVUS analyses were performed using computerized planimetry software (EchoPlaque 3.0; Indec Systems, Santa Clara, CA, USA).
Study endpoints
The primary endpoint was in-stent late lumen loss at 9-month angiography follow-up determined by QCA. Secondary endpoints were in-segment late lumen loss, binary restenosis rate, death, MI, TLR, target vessel revascularization (TVR), and stent thrombosis during 9-month follow-up.
Definition
MI was defined as chest pain and increased creatine kinase-myocardial band level to more than twice the upper limit of the normal range with or without a Q-wave on electrocardiography. Binary restenosis was defined as luminal narrowing of more than 50%, and TLR and TVR were defined as repeated PCI or bypass surgery of a previously stented site or vessel, respectively. Stent thrombosis was defined using the Academic Research Consortium definition.12)
Statistical analysis
This study was a non-inferiority trial of the Genoss DES™ stent for in-stent late lumen loss at 9-month angiography follow-up compared to the Promus Element™ stent. We assumed an in-stent late lumen loss of 0.20±0.28 mm in the Promus Element™ stent group based on prior study.13) We calculated 35 patients per group, using a one side α level 0.025 and non-inferiority margin of 0.19 mm with a statistical power of 80%. Assuming approximately 10% of patients would drop out, we calculated 40 patients for each group.
Data are presented as mean±standard deviations for continuous variables, and frequency (percentage) for categorical variables. Comparison of continuous variables was performed using Student's t-test, and χ2 or Fisher's exact tests were used for categorical variables. A p value <0.025 was considered statistically significant for the primary endpoint, and <0.050 for the secondary endpoints. Statistical analyses were performed using PASW software (version 18.0; SPSS Inc., Chicago, IL, USA).
RESULTS
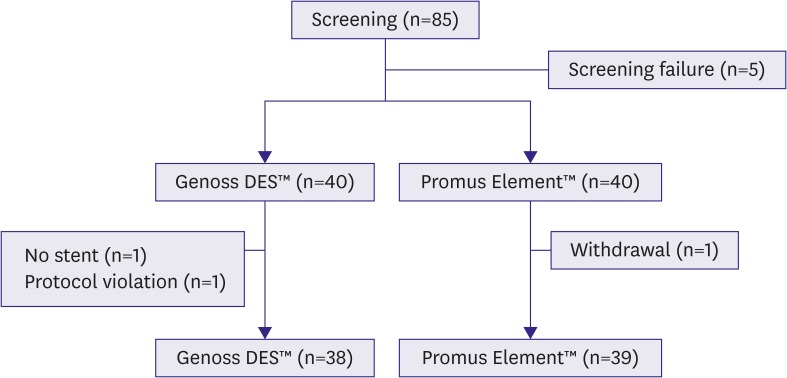
Between March 2013 and April 2015, 85 patients were screened for enrollment in the study. Five patients did not meet inclusion criteria and a total of 80 patients were randomly assigned to the Genoss DES™ or Promus Element™ group at a 1:1 ratio. In the Genoss DES™ group, 2 patients were excluded as one patient did not receive stent implantation and the other patient had a protocol violation. In the Promus Element™ group, 1 patient was excluded owing to the patient's withdrawal from the study (Figure 1).
Figure 1.
Flow chart of patient enrollment.
DES = drug-eluting stent.
Baseline clinical and angiographic characteristics are shown in Tables 1 and 2, respectively. There were no significant differences in clinical characteristics including age and clinical diagnosis between the 2 groups (Table 1). Target vessels were not significantly different, but the left anterior descending artery was targeted more often in the Promus Element™ group. Stent overlap was performed in 8 (21.1%) patients in the Genoss DES™ group and in 3 (7.7%) patients in the Promus Element™ group (Table 2).
Table 1. Baseline clinical characteristics.
| Genoss DES™ (n=38) | Promus Element™ (n=39) | p | ||
|---|---|---|---|---|
| Age (years) | 64±8 | 63±8 | 0.591 | |
| Male | 31 (81.6) | 31 (79.5) | 1.000 | |
| Diabetes | 12 (31.6) | 12 (30.8) | 1.000 | |
| Hypertension | 24 (63.2) | 29 (74.4) | 0.332 | |
| Hyperlipidemia | 15 (39.5) | 13 (33.3) | 0.640 | |
| Current smoker | 9 (23.7) | 13 (33.3) | 0.451 | |
| Diagnosis | 0.549 | |||
| Stable angina | 17 (44.7) | 19 (48.7) | ||
| Unstable angina | 21 (55.3) | 19 (48.7) | ||
| Silent ischemia | 0 | 1 (2.6) | ||
Data are shown as mean±standard deviation or number (%).
DES = drug-eluting stent.
Table 2. Angiographic and procedural characteristics.
| Genoss DES™ (n=38) | Promus Element™ (n=39) | p | ||
|---|---|---|---|---|
| Target vessel | 0.068 | |||
| Left anterior descending | 18 (47.3) | 26 (66.7) | ||
| Left circumflex | 4 (10.5) | 6 (15.4) | ||
| Right coronary | 16 (42.1) | 7 (17.9) | ||
| AHA/ACC classification | 0.089 | |||
| A | 4 (10.5) | 7 (17.9) | ||
| B1 | 6 (15.8) | 14 (35.9) | ||
| B2 | 10 (26.3) | 8 (20.5) | ||
| C | 18 (47.4) | 10 (25.6) | ||
| Stent diameter (mm) | 3.14±0.26 | 3.15±0.30 | 0.970 | |
| Stent length (mm) | 25.5±8.6 | 24.1±5.0 | 0.395 | |
| Mean stent number | 1.2 | 1.1 | 0.099 | |
| Stent overlap | 8 (21.1) | 3 (7.7) | 0.114 | |
Data are shown as mean±standard deviation or number (%).
ACC = American College of Cardiology; AHA = American Heart Association; DES = drug-eluting stent.
Angiographic follow-up at 9 months was performed for all patients, and IVUS follow-up was performed in 35 (92%) patients in the Genoss DES™ group and in 37 (95%) patients in the Promus Element™ group. QCA results are shown in Table 3. There were no significant differences in RVD. For the primary endpoint, in-stent late lumen loss at 9 months was not significantly different between the groups (0.11±0.25 mm for Genoss DES™ vs. 0.16±0.43 mm for Promus Element™, p=0.567) nor was in-segment late lumen loss (0.11±0.26 mm for Genoss DES™ vs. 0.15±0.43 mm for Promus Element™, p=0.558). Angiographic restenosis at 9 months was observed in 1 (2.6%) patient in the Genoss DES™ group and in 2 (5.1%) patients in the Promus Element™ group (p=1.000). IVUS minimal lumen area after stenting during the index procedure (6.95±1.98 vs. 7.47±2.47 mm2, p=0.272) and after 9 months (6.95±1.98 vs. 7.29±2.34 mm2, p=0.508) were not significantly different (Table 4). For clinical outcomes, there were no MI or stent thrombosis events during 9-month follow-up. One death occurred in the Genoss DES™ group due to aggravated renal function 6 months after the index procedure. However, the rate of death, MI, TLR, and TVR at 9 months were not significantly different (Table 5).
Table 3. QCA analysis.
| Genoss DES™ (n=38) | Promus Element™ (n=39) | p | |||
|---|---|---|---|---|---|
| RVD (mm) | |||||
| Pre-procedure | 3.24±0.41 | 3.12±0.43 | 0.199 | ||
| Post-procedure | 3.30±0.30 | 3.33±0.30 | 0.652 | ||
| 9-month follow-up | 3.17±0.35 | 3.16±0.39 | 0.932 | ||
| Minimal lumen diameter (mm) | |||||
| In-stent | |||||
| Pre-procedure | NA | NA | NA | ||
| Post-procedure | 2.95±0.35 | 3.03±0.30 | 0.269 | ||
| 9-month follow-up | 2.84±0.42 | 2.87±0.56 | 0.743 | ||
| In-segment | |||||
| Pre-procedure | 0.83±0.29 | 0.88±0.45 | 0.572 | ||
| Post-procedure | 2.90±0.27 | 3.00±0.36 | 0.159 | ||
| 9-month follow-up | 2.79±0.40 | 2.85±0.56 | 0.615 | ||
| DS % | |||||
| In-stent | |||||
| Pre-procedure | NA | NA | NA | ||
| Post-procedure | 10.6±3.48 | 9.16±4.85 | 0.140 | ||
| 9-month follow-up | 14.18±9.31 | 13.73±14.30 | 0.871 | ||
| In-segment | |||||
| Pre-procedure | 74.41±8.62 | 72.65±12.14 | 0.465 | ||
| Post-procedure | 12.10±3.93 | 10.12±4.94 | 0.056 | ||
| 9-month follow-up | 15.46±9.39 | 14.54±14.08 | 0.738 | ||
| Acute gain (mm) | |||||
| In-stent | 1.79±0.40 | 1.82±0.36 | 0.742 | ||
| In-segment | 1.74±0.39 | 1.78±0.38 | 0.579 | ||
| Late loss (mm) | |||||
| In-stent | 0.11±0.25 | 0.16±0.43 | 0.567 | ||
| In-segment | 0.11±0.26 | 0.15±0.43 | 0.558 | ||
| Lesion length (mm) | 23.8±8.1 | 22.8±4.9 | 0.531 | ||
| Restenosis | 1 (2.6) | 2 (5.1) | 1.000 | ||
Data are shown as mean±standard deviation or number (%).
DES = drug-eluting stent; DS = diameter stenosis; NA = not available; QCA = quantitative coronary angiography; RVD = reference vessel diameter.
Table 4. IVUS analysis results.
| Genoss DES™ (n=35) | Promus Element™ (n=37) | p | ||
|---|---|---|---|---|
| Minimal lumen area (mm2) | ||||
| Post-procedure | 6.71±1.74 | 7.28±2.41 | 0.255 | |
| 9-month follow-up | 6.30±1.78 | 6.72±2.28 | 0.387 | |
| Minimal stent area (mm2) | ||||
| Post-procedure | 6.90±1.77 | 7.47±2.47 | 0.272 | |
| 9-month follow-up | 6.95±1.98 | 7.29±2.34 | 0.508 | |
| EEM | ||||
| Post-procedure | 14.87±3.23 | 14.91±4.40 | 0.963 | |
| 9-month follow-up | 15.42±3.27 | 14.74±3.90 | 0.423 | |
Data are shown as mean±standard deviation.
DES = drug-eluting stent; EEM = external elastic membrane; IVUS = intravascular ultrasound.
Table 5. Clinical outcomes.
| Genoss DES™ (n=38) | Promus Element™ (n=39) | p | ||
|---|---|---|---|---|
| Death | 1 (2.6) | 0 | 0.494 | |
| Cardiac | 0 | 0 | 1.000 | |
| Non-cardiac | 1 (2.6) | 0 | 0.494 | |
| MI | 0 | 0 | 1.000 | |
| TLR | 1 (2.6) | 1 (2.6) | 1.000 | |
| TVR | 3 (7.9) | 1 (2.6) | 0.358 | |
| Stent thrombosis | 0 | 0 | 1.000 | |
Data are shown as number (%).
DES = drug-eluting stent; MI = myocardial infarction; TLR = target lesion revascularization; TVR = target vessel revascularization.
DISCUSSION
This prospective, multicenter, randomized first-in-patient study showed that in-stent late lumen loss of Genoss DES™ stents was not inferior to Promus Element™ stents as determined by QCA at 9-month angiography follow-up. Moreover, there were no differences in clinical outcomes and no stent thrombosis during 9 months of follow-up.
The Genoss DES™ stent is the first DES made in Korea. It consists of a cobalt-chromium alloy stent platform, an abluminal coating of bioresorbable polymers, and a drug-eluting coating with sirolimus. Sirolimus is an anti-proliferative and anti-inflammatory agent that inhibits progression from the G1 to the S phase of the cell cycle. Coronary DES using sirolimus has demonstrated good clinical outcomes.4),14)
For the Genoss DES™ stent, animal studies using pigs were performed to determine the optimal dose of the drug, comparing high and low doses of sirolimus and paclitaxel (unpublished data). Consequently, sirolimus was loaded on the stent at a concentration of 1.15 µg/mm2. The stent was designed to release approximately 70% of the total drug amount within 30 days after implantation. To control the drug release profile, it possesses an ultra-thin (4 µm) bioresorbable layer applied to an abluminal stent surface. This bioresorbable layer consists of a proprietary blend of PLGA and PLA, which are completely resorbable within 6 months, to reduce the possibility of stent thrombosis due to polymer hypersensitivity. In addition, the cobalt-chromium alloy stent platform reduces strut thickness and maximizes flexibility.
In-stent late luminal loss at 9 months with the Genoss DES™ stent was 0.11±0.25 mm, comparable with previous DES trials (Table 6). Moreover, this study included a number of patients at high risk of unstable angina or complex lesions. The proportion of patients with unstable angina was around 50% in both groups and that of American Heart Association (AHA)/American College of Cardiology (ACC) type C lesions was 47.4% for the Genoss DES™ group and 25.6% for the Promus Element™ group. Angiographic in-stent restenosis was observed in only 2.6% of patients in the Genoss DES™ group, even though stent overlap was permitted such that mean lesion length was very high (25.5 mm for the Genoss DES™ group) compared with previous studies (Table 6). Considering patient and angiographic characteristics, in-stent late lumen loss of the Genoss DES™ stent at 9-month follow-up was acceptable in real clinical practice.
Table 6. Comparison of angiographic outcomes with previous studies.
| In-stent late loss (mm) | Lesion length (mm) | |
|---|---|---|
| Platinum QCA13) | 0.17±0.25 | 15.4±7.0 |
| Nobori 115) | 0.11±0.30 | 10.56 |
| SPIRIT I16) | 0.10±0.21 | 10.1±2.6 |
| RESOLUTE17) | 0.22±0.27 | 15.61±6.13 |
| Genoss DES™ (current study) | 0.11±0.25 | 25.5±8.6 |
Data are shown as mean±standard deviation.
DES = drug-eluting stent; QCA = quantitative coronary angiography.
For clinical outcomes, there were no differences in death, MI, TLR, or TVR between the 2 groups. TLR occurred in only 2.6% of patients in both groups at 9-month angiography follow-up. In the Genoss DES™ group, 2 cases of TVR without TLR occurred, which were new lesions in the proximal portions of the right coronary artery independent of previously stented sites. Although not statistically significant, the stent overlap rate at the target lesion was higher in the Genoss DES™ group than in the Promus Element™ group (21.1% vs. 7.7%). However, the clinical event rate of the Genoss DES™ group was very low. The Genoss DES™ stent did not develop stent thrombosis during the 9-month follow-up despite complex lesion characteristics.
This study has several limitations. For assumptions about sample size, the non-inferiority margin was relatively high and the number of patients was small. This made the statistical power weak. However, the Genoss DES™ stent showed excellent in-stent late lumen loss compared to current second-generation DESs. In addition, as this is a first-in-patient trial, more studies with larger populations should be conducted in real clinical practice.
In conclusion, this first-in-patient study of the Genoss DES™ stent showed excellent angiographic outcomes for in-stent late lumen loss and major adverse cardiac events during the 9-month follow-up.
Footnotes
Funding: This study was supported by Genoss Company Limited (Suwon, Korea).
Conflict of Interest: Tahk SJ received research grant from Genoss Compnary Limitied, and other investigators did not have any financial relationships or any other biases or conflicts of interest related with this study.
- Conceptualization: Tahk SJ, Yang HM.
- Data curation: Yoon J, Kim HS, Chang K, Lee SH, Ahn SG, Youn YJ, Lee JW, Koo BK, Park KW, Yang HM, Han JK, Seung KB, Chung WS, Kim PJ, Koh YS, Park HJ.
- Formal analysis: Tahk SJ, Yang HM.
- Funding acquisition: Yoon J, Kim HS, Chang K, Lee SH, Ahn SG, Youn YJ, Lee JW, Koo BK, Park KW, Yang HM, Han JK, Seung KB, Chung WS, Kim PJ, Koh YS, Park HJ.
- Validation: Yoon J, Kim HS, Chang K, Lee SH, Ahn SG, Youn YJ, Lee JW, Koo BK, Park KW, Yang HM, Han JK, Seung KB, Chung WS, Kim PJ, Koh YS, Park HJ.
- Writing - original draft: Tahk SJ, Yang HM.
References
- 1.Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 2.Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 5.Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 6.Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 7.Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115:1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 8.Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006;47:175–181. doi: 10.1016/j.jacc.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 9.Zhang YJ, Ye F, Iqbal J, et al. NOBORI™ biodegradable-polymer biolimus-eluting stent versus durable-polymer drug-eluting stents: a meta-analysis. Int J Cardiol. 2014;174:151–153. doi: 10.1016/j.ijcard.2014.03.167. [DOI] [PubMed] [Google Scholar]
- 10.Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33:1214–1222. doi: 10.1093/eurheartj/ehs086. [DOI] [PubMed] [Google Scholar]
- 11.Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 12.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 13.Meredith IT, Whitbourn R, Scott D, et al. PLATINUM QCA: a prospective, multicentre study assessing clinical, angiographic, and intravascular ultrasound outcomes with the novel platinum chromium thin-strut PROMUS Element everolimus-eluting stent in de novo coronary stenoses. EuroIntervention. 2011;7:84–90. doi: 10.4244/EIJV7I1A15. [DOI] [PubMed] [Google Scholar]
- 14.Windecker S, Serruys PW, Wandel S, et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372:1163–1173. doi: 10.1016/S0140-6736(08)61244-1. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier B, Silber S, Park SJ, et al. Randomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberté paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the NOBORI 1 trial--Phase 2. Circ Cardiovasc Interv. 2009;2:188–195. doi: 10.1161/CIRCINTERVENTIONS.108.823443. [DOI] [PubMed] [Google Scholar]
- 16.Serruys PW, Ong AT, Piek JJ, et al. A randomized comparison of a durable polymer Everolimus-eluting stent with a bare metal coronary stent: the SPIRIT first trial. EuroIntervention. 2005;1:58–65. [PubMed] [Google Scholar]
- 17.Meredith IT, Worthley S, Whitbourn R, et al. Clinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trial. JACC Cardiovasc Interv. 2009;2:977–985. doi: 10.1016/j.jcin.2009.07.007. [DOI] [PubMed] [Google Scholar]