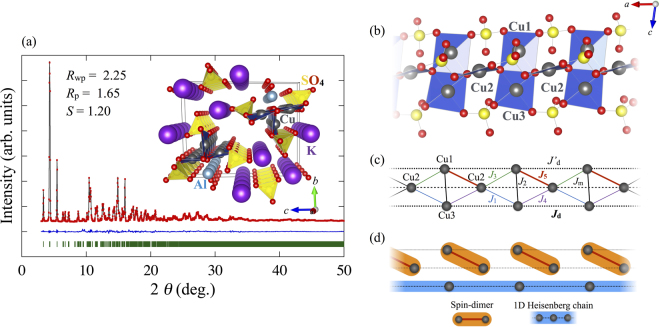
Figure 1.
(a) Synchrotron XRD intensity pattern (filled red circles) observed for K3Cu3AlO2(SO4)4 at room temperature, the result of Rietveld refinement using the computer program RIETAN-FP22 (black solid line), and difference between the calculated and observed intensities (blue solid line). The green vertical bars indicate the position of Bragg reflection peaks. The inset shows the crystal structure of K3Cu3AlO2(SO4)4 featuring a large inter-chain spacing. (b) The diamond chain of K3Cu3AlO2(SO4)4, which consists of Cu2+ ions (grey spheres) along the a-axis with nearby oxygen (red spheres) and sulfur ions (yellow spheres). (c) Effective spin model of K3Cu3AlO2(SO4)4 with the nearest-neighbor exchange couplings J i (i = 1 to 5), and the next nearest-neighbor exchange couplings of J m, J d, and . (d) Spin configuration of the ground state for K3Cu3AlO2(SO4)4.