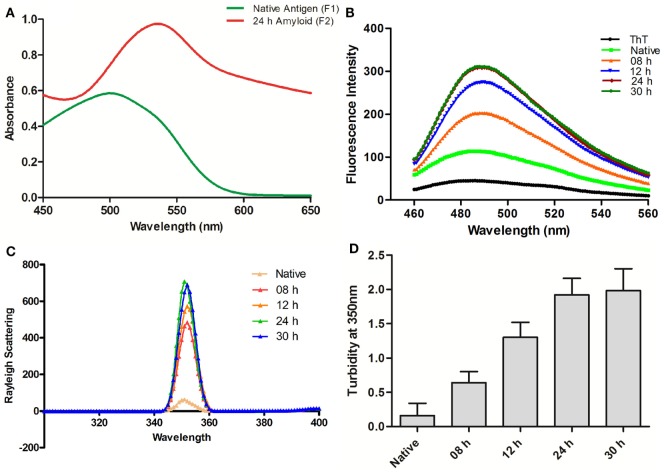
Figure 1.
Biophysical characterization of Ag85B aggregates. (A) Absorption spectra of Congo Red dye bound to aggregated amyloidal Ag85B. The characteristic spectral “Red Shift” confirms the presence of β-sheet rich amyloidal core. (B) Amyloidal aggregate formation as monitored by Thioflavin-T fluorescence spectroscopy. Gradual increase in ThT emission intensities, as obtained by excitation at 450 nm and emission in the range of 460–560 nm, an indication for sequential maturation of Ag85B fibril at various time points. (C) Rayleigh Scattering characteristic at 350 nm for Ag85B aggregates. Data pertaining to various samples obtained from simulated reaction process at various time points confirm that the extended agitation gradually induced aggregation of soluble Ag85B. (D) Turbidity profile of synthesized aggregates. Various aggregated forms of Ag85B (formed at various incubation stages) were also scanned for increase in the turbidity of protein suspension. Error bars exhibit ±standard errors of means (±SEM). Three independent experiments were performed for each sample and data are representative of at least two independent experiments with similar observations.