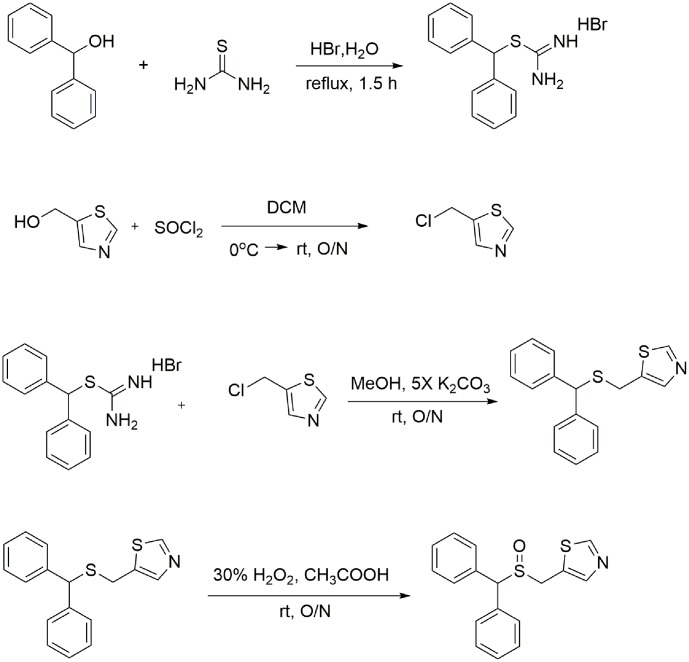
FIGURE 1.
Synthesis of the CE-123 race mate. In the first step diphenylmethanol is reacted with thiourea to yield thiouronium salt. In the second step thiazol-5yl-methanol is converted to 5-(chloromethyl) thiazole. The third step is the alkylation reaction of thiouronium salt with 5-(chloromethyl) thiazole to yield 5-((benzhydrylthio) methyl) thiazole (un-oxidized CE-123 precursor). Finally, 5-((benzhydrylthio)methyl) thiazole is oxidized with 30% H2O2 in gl. acetic acid to yield the final product 5-((benzhydrylsulfinyl)methyl) thiazole (CE-123).