Figure 2.
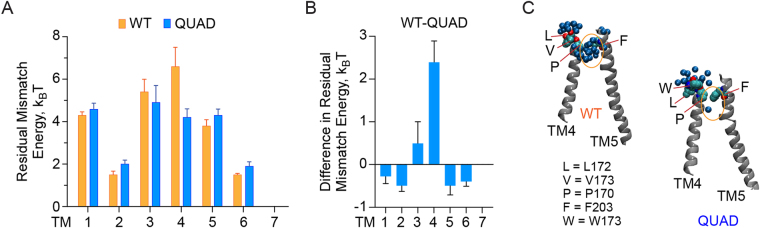
Residual hydrophobic mismatch (RHM) at TM helices in WT and QUAD opsin. (A) RHM energies were calculated at TM helices for WT and QUAD opsin, using microsecond-scale atomistic MD simulations (see Methods) in an explicit 9:1 mixture of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-(1′-glycerol) (POPG); error bars represent standard deviation of RHM measurements carried out on overlapping time intervals of the MD trajectory. (B) Difference in RHM energies between WT and QUAD opsin calculated from the data in panel A. The difference in RHM at the TM4 helix was statistically highly significant (p value < 0.002 from unpaired t-test). (C) Final snapshots from the simulations illustrating the source of RHM energies at TM4. Shown are TM4 and TM5 (in cartoon), and amino acid residues as indicated (van der Waals representation). Dark blue spheres are water oxygens within 5 Å of these residues. Water accumulation at the exoplasmic ends of TM4 and TM5 in WT opsin (region within orange oval) breaks hydrophobic contacts between P170 and F203, resulting in a large RHM at TM4. This hydrophobic contact is intact in the QUAD protein, thus reducing the RHM.