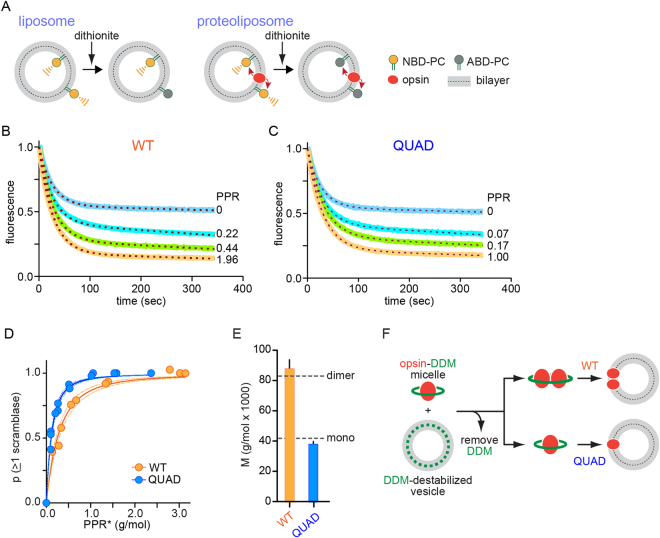
Figure 5.
QUAD opsin scrambles lipids as a monomer. (A) Schematic representation of the fluorescence-based scramblase activity assay. (B) Representative fluorescence traces of scrambling by WT opsin, reconstituted at different protein/phospholipid ratios (PPR) into POPC:POPG (9:1) vesicles containing a trace amount of NBD-PC. (C) As in panel B, for QUAD opsin. (D) The extent of fluorescence reduction in the scramblase assay was determined for vesicles reconstituted with QUAD and WT opsin over a range of PPR values, 0–3 g/mol and the data were transformed into plots of p(≥1) scramblase (the probability of a vesicle having at least one scramblase) vs PPR* (related to measured PPR, see Experimental Procedures). The solid lines are data fits (Poisson analysis, see Experimental Procedures and Table 2), and the dashed lines are the 95% confidence interval for the fits. (E) Molar mass of the functionally reconstituted scramblase deduced from the data shown in panel D (see Table 2). (F) Schematic illustration showing that whereas both WT and QUAD opsin are monomers when added to DDM-destabilized phospholipid vesicles (based on Fig. 4), WT opsin dimerizes (multimerization is not shown here for simplicity) en route to reconstitution whereas QUAD opsin reconstitutes as a monomer (based on this figure, panel E). Direct evidence for dimerization of WT opsin during detergent withdrawal was previously obtained through co-immunoprecipitation studies13.