Figure 2.
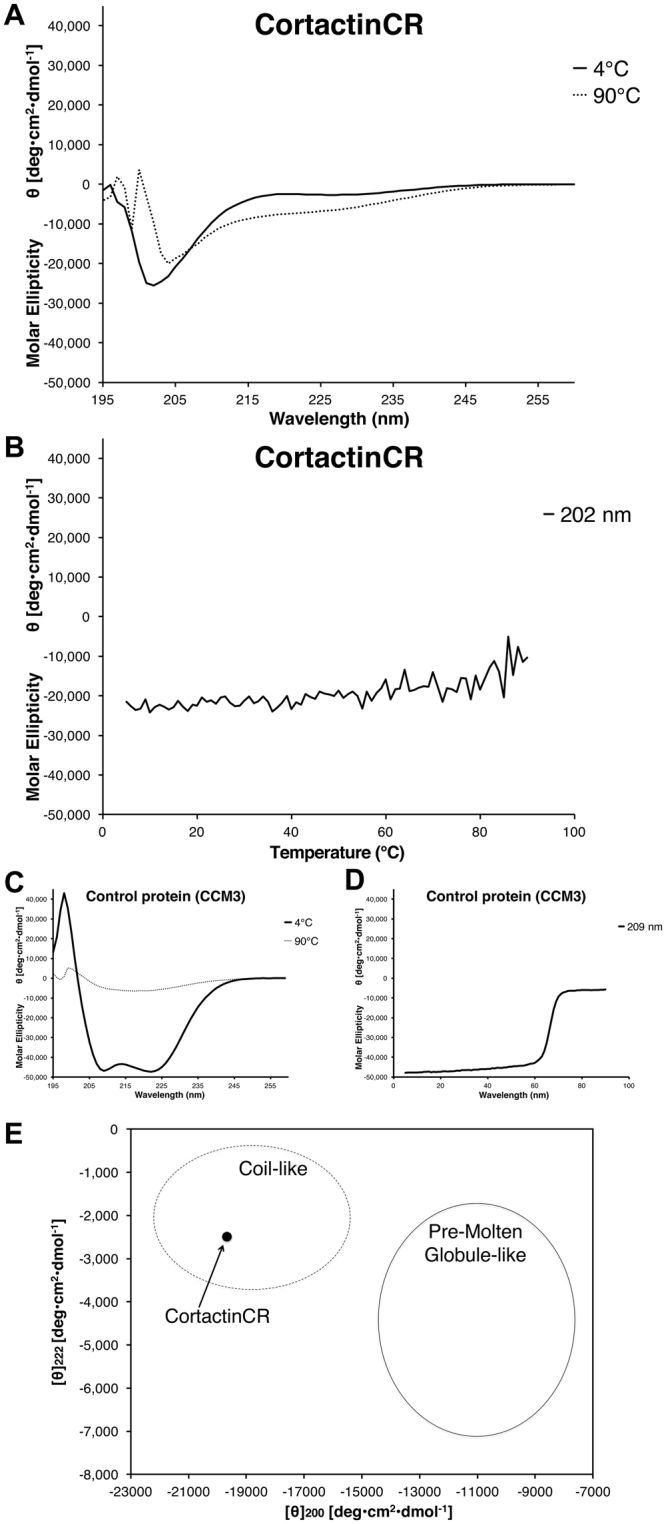
Circular Dichroism for the cortactin repeat domain. (A) Far UV CD spectrum for cortactinCR at 4 °C (solid line) shows a negative peak at 202 nm, but no features that could be interpreted as α-helical or β-sheet. A red shift of ~2 nm occurs on increase in temperature from 4 °C to 90 °C (dashed line). (B) Temperature dependence of molar ellipticity from 4 °C to 90 °C monitored at 202 nm does not show a melting point typical of folded proteins. (C) CD spectra for a well folded α-helical control protein, CCM3, at 4 °C and 90 °C. Secondary structure is lost at 90 °C. (D) Melting point analysis for CCM3 shows that this control protein melts between 60 °C and 70 °C. (E) Analysis of [θ]222 vs [θ]200 for cortactinCR. Plotting [θ]222 vs [θ]200 suggests that cortactinCR is falls into the coil-like unfolded protein class and not the pre-molten globule class. Analysis based on23.
