Abstract
Objective
Cigarette smoking is a risk factor for earlier menopause. Animal studies show that in-utero smoke exposure is toxic to developing ovaries. Our aim was to evaluate whether in-utero smoke exposed women reach menopause earlier compared to non-exposed women.
Methods
This is a cohort study within the Avon Longitudinal Study of Parents and Children (ALSPAC) in which pregnant women (participants) were followed from 1991/1992 until 2010. Participant characteristics for the current analysis were obtained from obstetric records and from annual follow-up questionnaires. When not available, age at natural menopause was estimated by age at filling in the questionnaire minus one year. Cox proportional hazards modeling was used to estimate hazard ratios of menopause for in-utero exposed and non-exposed participants.
Results
There were 695/2852 postmenopausal participants, of whom 466 had natural menopause , 117 had hormonal therapy and 112 had surgical menopause. Age at natural menopause was 50.6±3.7 years. Of all participants, 20.2% (577/2852) were exposed to smoke in-utero. Participants who were in-utero exposed but were not smokers did not have higher hazards of menopause (adjusted HR 0.92, 95%CI 0.72-1.18) while participants who were ever smokers (current or previous) and were in-utero exposed (adjusted HR 1.41 95%CI 1.01-1.95) or were ever smoker but not exposed (adjusted HR 1.24 95% CI 1.00-1.53) did.
Conclusion
In-utero smoke exposure was not associated with earlier menopause but the effect of in-utero smoke exposure was modified by the smoking habits of the participants themselves, increasing the risk for smoker who were in-utero exposed.
Keywords: menopause, final menstrual period, cigarette smoking, prenatal exposure, ALSPAC
Introduction
Menopause occurs when the ovarian follicle pool is depleted to a minimum threshold 1. The depletion of these follicles is a process that starts before birth and it continues until menopause2. The ovarian reserve, i.e. the population of non-growing follicles (NGF), is established prenatally; at 20 weeks post conception the population of NGF reaches a peak size3 and some studies suggest that age at menopause is linked to the size of this initial cohort of NGF4,5. Since this follicle pool is prenatally determined, early life events that are harmful to germ cells, can diminish the initial finite follicle pool a woman is born with6 and thus might influence timing of menopause.
Women who are smokers reach menopause approximately one year earlier compared to non-smokers7,8. Components of cigarette smoke, such as polycyclic aromatic hydrocarbons (PAH) and cadmium are involved in pathways leading to follicle loss, either via aryl hydrocarbon receptors in granulosa cells9 or via inhibition of gap-junctions of oocytes and granulosa cells10. Female germ cells are regarded to be especially vulnerable to cigarette smoke exposure during critical periods of primordial follicles development, when mitotic divisions of oogonia occur11. Mice pups whose pregnant mothers were nasally exposed to mainstream cigarette smoke during pregnancy showed half the number of primordial and primary follicles when compared to controls12. In human, 29 fetal ovaries obtained after legal abortions (aged 38-64 days post conception), in-utero smoke exposure was associated with a non-significant decrease in the number of oogonias13. These studies suggest that women who are in-utero exposed to cigarette smoke are born with a smaller ovarian reserve compared to non-exposed women, and therefore, might exhaust their follicle pool quicker, reaching menopause earlier.
However, epidemiological studies have not indisputably confirmed nor rejected this hypothesis. Therefore, this study aimed to determine whether there is an association between in-utero smoke exposure and age at menopause in a longitudinal cohort of women whose menopause age was assessed 18 years after entry in the study. We have hypothesized that in-utero smoke exposed women reach menopause earlier compared to unexposed women.
Materials and Methods
Participants
This is a cohort study within the ongoing Avon Longitudinal Study of Parents and Children (ALSPAC) cohort, which was designed to identify environmental and genetic characteristics that can influence health and development in parents and children14–16. Pregnant women (participants) residents of the Avon County, UK, expected to deliver between April 1st 1991 and December 31st 1992 were included in the study. Participant’s characteristics for the current analyses were obtained from obstetrical records and from repeated annual follow-up questionnaires. Initially, 14,541 pregnant women (approximately 90% of pregnancies in the study area for enrollment period) were enrolled in the study. In 2010, 18 years after inclusion in the study, questionnaires were sent to 9028 participants who were still active in the cohort; 4175 (46.2%) of them returned the questionnaires and these participants were used in the current study. The main risk factor, in-utero smoke exposure, was obtained from questionnaires at baseline. Participants were asked “did your mother smoke?” and “did your mother smoke when pregnant of you (participant)?” and participants could answer in three different ways: “yes”, “no” and “I don’t know”. These questions were asked to participant again 8 years later. In order to evaluate misclassification and recall bias for in-utero smoke exposure, two analyses were performed. The first analysis evaluated whether in-utero smoke exposed participants were born with a lower birth weight compared to participants who were not exposed. The second analysis compared answers for in-utero smoke exposure given by the participants for two time points: at enrolment and 8 years later. Menopause status was obtained from questionnaires sent 18 years after baseline. Participants were asked: “in the last 12 months, have you had a period or menstrual bleeding?” and “what was your age at last menstrual bleeding? Please note that the study website contains details of all the data that is available through a fully searchable data dictionary available at http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees available at http://www.bristol.ac.uk/alspac/researchers/research-ethics
Statistical analysis
Participants with inconsistent or missing information on age at surgery, start of HT (hormone therapy) use, or no age at oncological treatment (postmenopausal due to chemotherapy or radiotherapy) were excluded. Participants who reported “other reasons” for menopause in the questionnaire were excluded. Participants with a history of ever using HT or having ovarian/uterine surgical removal before natural menopause were censored at the age of the event. Premenopausal women using HT were also censored at age when they started treatment. For participants who reported being postmenopausal but did not provide age at last menstrual bleeding, age at natural menopause was calculated from age at completion of the questionnaire minus one year.
Differences in characteristics of participants who were in-utero exposed and participants who were not exposed were tested using unpaired Student T-tests, Mann-Whitney or Chi-Square tests as appropriate. Cox proportional hazards modeling was used to estimate the crude and adjusted effect of in-utero smoke exposure on the age at menopause. Results were adjusted for factors associated with both in-utero smoke exposure and age at menopause. Factors that were different between groups but not associated with age at menopause were not included in the adjusted analyses. A hazard ratio (HR) higher than one indicates that in-utero smoke exposure is associated with a higher probability of reaching menopause compared to non-exposed during any given time period. Violation of the proportional hazards assumption was evaluated with Schoenfeld residuals test and the assumption was met (p-value= 0.40).
BMI included in the analyses was assessed at the enrolment period. Data analyzed on participants smoking history included exposure by paternal and/or partner smoking and smoking of participants themselves. Smoking habits of participants were quantified by pack years using data from the follow-up questionnaires (18 assessments in total). Exposure years were calculated by subtracting the age at the assessment point from the age when the participant started smoking. This number was multiplied by the number of cigarettes smoked per day divided by number of cigarettes per pack ([ age at assessment point-age started smoking]*[ number of cigarettes per day]/20). This was performed for each questionnaire interval and summed up. Participants were then categorized into heavy smokers (>25 pack years), moderate (>10 and ≤25 pack years), light (< 10 pack years) and non-smokers. At time point 18 years after enrolment, participants were asked if they are smokers. Those who answered “yes” were classified as current smokers. Previous smokers are the non current smokers with pack years different than zero. Never smokers are participants with zero pack years.Ever smokers include participants who are current or previous smokers.
In order to verify a possible interaction between smoking habit of participants and in-utero smoke exposure, an interaction term was created with the product term from these two factors ( in-utero smoke exposure and smoking of participants themselves), including pack years as a continuous factor. The participants were then stratified into four groups: 1. exposed in-utero and ever smoker, 2. exposed in-utero and non-smoker, 3. not exposed in-utero and ever smoker and 4. not exposed in-utero and non-smoker. The latter served as the reference group.
Results
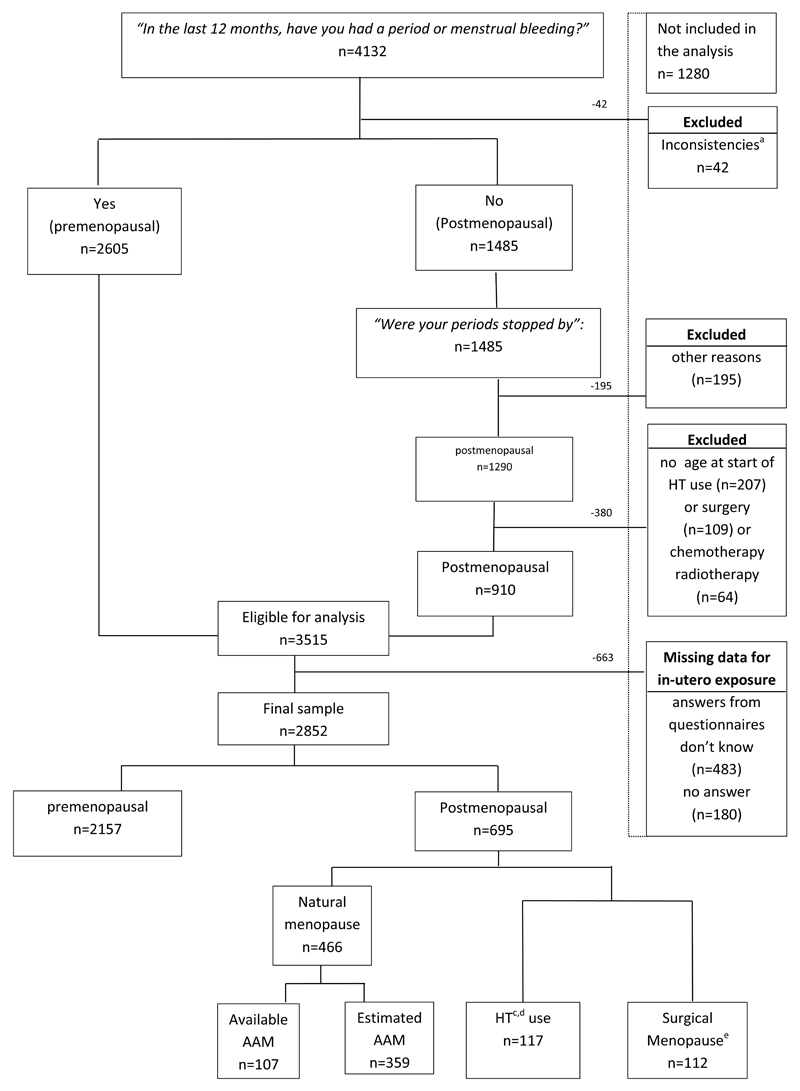
From the questionnaires available, 4132/4175 participants provided information on menopause status. There were 7 participants missing data, 36 duplicates participants (twin pregnancies) and 3515 participants were eligible for analysis after exclusions (see figure I). Out of these, 663/3515 (18.9%) had missing information about in-utero smoke exposure; 483/663 answered I don’t know” and 180/663 did not answer the question. There were 2157/2852 (75.6%) premenopausal participants and 695/2852 (24.4%) postmenopausal participants. Natural menopause was reported by 466/695 (67.1%) participants of which 359/466 (77.0%) did not provide age at menopause; 112/695 (16.1%) participants had surgical menopause and 117/695 (16.8%) participants used HT. Participants who reported menopause due to other reasons were excluded. Women with missing information on age at surgery and/or start of HT or, no age at oncological treatment (postmenopausal due to chemotherapy or radiotherapy) were excluded (see figure I).
Figure I.
Flow chart of participants.
Abbreviations: AAM age at menopause, HT (Hormone therapy). a. Inconsistencies are participants that had more than one answer to menopausal status. b.42 women were premenopausal and using HT. c. These participants are censored at the age they started using HT or age at surgery
For the participants who had surgery before menopause, 39 participants had hysterectomy and bilateral oophorectomy, 51 had hysterectomy, 6 had hysterectomy and unilateral oophorectomy, 2 had bilateral oophorectomy and 14 had unilateral oophorectomy.
The kappa level of agreement comparing answers from in-utero smoke exposure for two time points (at enrolment and 8 years later, n=1032) was 0.91 (p-value 0.001) and there was a disagreement of 5% between answers.
Table I shows characteristics of the participants. Of all participants, 20.2% (577/2852) were smoke exposed in-utero and 79.8% (2775/2852) were not exposed. In-utero smoke exposed participants were born with a significant lower birth. Compared to participants who were not exposed, participants who were exposed in-utero were younger, had a higher BMI, were younger when they started using oral contraceptives (OC) and, when they had their first pregnancy. There were more current and previous smokers among exposed participants and there was higher passive smoke exposure due to smoking of fathers and partners. In-utero exposed participants also started smoking at a younger age compared to unexposed participants.
Table I.
Characteristics of participants
| No. | In-utero exposed n=577/2852 (20.2%) |
Not exposed n=2275/2852 (79.8%) |
p-value | |
|---|---|---|---|---|
| Agea | 2852 | 46.9±4.7 | 47.5±4.3 | 0.003b |
| Birth weight | 1887 | 3129.7±602.0 | 3333.8±559.0 | <0.001b |
| BMI | 2798 | 23.1±3.7 | 22.5±3.4 | <0.001c |
| Underweight | 251 | 52 (9.2) | 199 (8.9) | |
| Normal | 2069 | 401 (70.6) | 1668 (74.8) | |
| Overweight | 371 | 85 (15.0) | 286 (12.8) | |
| Obese | 107 | 30 (5.3) | 77 (3.5) | |
| Age at menarche | 2589 | 12.9±1.5 | 12.9±1.4 | 0.59b |
| 8-11 years | 454 | 100 (18.7) | 354 (17.2) | |
| 12-14 | 1814 | 367 (68.7) | 1447 (70.4) | |
| 15 or more | 321 | 67 (12.5) | 254 (12.4) | |
| Regular menstrual periodse | 2813 | 453/567 (80.0) | 1759/2246 (78.3) | 0.17d |
| Cycle length (days)e | 2140 | 27.9±3.5 | 28.0±3.6 | 0.90c |
| 20 or less | 25 | 4 (0.9) | 21 (1.2) | |
| 21-35 | 2100 | 431 (98.6) | 1669 (98.0) | |
| 36 or more | 15 | 2 (0.5) | 13 (0.8) | |
| Ever used OC pill | 2819 | 542/567 (95.6) | 2119/2252 (94.1) | 0.17d |
| Age at OC start | 2648 | 18.0±2.5 | 18.9±2.9 | <0.001c |
| Ever seen a doctor for possible infertility | 2816 | 68/568 (12.0) | 279/2248 (12.4) | 0.78d |
| Age at 1st pregnancy | 2852 | 25.1±5.0 | 26.5±4.6 | <0.001b |
| Parity | 0.17d | |||
| 1 | 2577 | 216/511 (42.3) | 804/2066 (38.9) | |
| 2 or more | 2577 | 295/511 (57.7) | 1262/2066 (61.1) | |
| Ever had ART | 2816 | 24/568 (4.2) | 70/2248 (3.1) | 0.19d |
| Ever had surgery | 2852 | 24/568 (4.2) | 70/2248 (3.1) | 0.19d |
| Ever used HT | 2852 | 23/577 (4.0) | 89/2275 (3.9) | 0.94d |
| Age start HT | 117 | 45.9±5.5 | 46.2±5.3 | 0.73c |
| Active smoking | <0.001d | |||
| Current | 2852 | 96/577 (16.6) | 192/2275 (8.4) | |
| Previous | 2852 | 111/577 (19.2) | 347/2275 (15.3) | |
| Never | 2852 | 370/577 (64.1) | 1736/2275 (76.3) | |
| Age started smoking | 715 | 16.3±2.2 | 17.1±2.8 | <0.001b |
| Pack yearsf | 715 | 12.3±9.1 | 7.9±8.5 | <0.001c |
| Heavy | 47 | 30.1±4.2 | 31.4±6.8 | |
| Moderate | 225 | 16.8±4.1 | 15.8±4.0 | |
| Light | 443 | 4.4±3.4 | 3.3±3.0 | |
| No smoker | 2116 | 0 | 0 | |
| Passive smoking | ||||
| Participant’s father smoker | 2794 | 498/568 (87.7) | 1453/2226 (65.3) | <0.001d |
| Participant’s partner smoker | 2793 | 198/568 (34.9) | 563/2225 (25.3) | <0.001d |
Abbreviations: BMI body max index, OC oral contraceptives, ART assisted reproductive technology, HT Hormone Therapy.
Age 18 years after start of the study.
T-test
Mann-Whitney U Test
Pearson’s Chi-Squared Test
information on menstrual period at baseline.
10 participants who answered being a smoker had 0 cumulative pack years and 21 participants who reported being smokers did not have cumulative number of cigarettes smoked.
Table II shows hazards ratios for menopause obtained from univariate analyses. A shorter cycle length, a younger age at start of OC, a younger age at first pregnancy, and having had ART were associated with earlier menopause. Being a smoker was associated with earlier menopause as were a younger age at start of smoking and being a heavy smoker.
Table II.
Core covariates and their association with age at menopause of participants
| Crude HR, 95%CI | p-value | |
|---|---|---|
| Birth weight | 1.00, 1.00-1.00 | 0.53 |
| BMIa | 1.01, 0.99-1.03 | 0.55 |
| Underweight | 0.88, 0.70-1.13 | 0.32 |
| Normal | reference | |
| Overweight | 1.09, 0.90-1.32 | 0.36 |
| Obese | 1.06, 0.76-1.47 | 0.74 |
| Age at menarche | 0.97, 0.92-1.01 | 0.15 |
| 8-11 years | 1.15, 0.96-1.38 | 0.13 |
| 12-14 | reference | |
| 15 or more | 0.94, 0.77-1.16 | 0.56 |
| Regular menstrual periodsa | ||
| Yes | reference | |
| No | 0.92, 0.78-1.09 | 0.35 |
| Cycle length (days)a | 0.96, 0.94-0.98 | <0.001 |
| 20 or less | 2.09, 1.04-4.21 | 0.04 |
| 21-35 | reference | |
| 36 or more | 0.47, 0.06-3.36 | 0.45 |
| Ever used OC | 0.89, 0.69-1.15 | 0.37 |
| Age 1st used OC | 0.95, 0.93-0.98 | <0.001 |
| Ever seen a doctor for possible infertility | 1.19, 1.00-1.41 | 0.05 |
| Age at 1st pregnancy | 0.98, 0.97-0.99 | <0.001 |
| Parity | ||
| 1 | 1.04, 0.90-1.20 | 0.62 |
| 2 or more | reference | |
| Ever had ART treatment | 1.37, 1.03-1.83 | 0.03 |
| Active smoking | ||
| Current | 1.45, 1.20-1.86 | <0.001 |
| Previous | 1.22, 1.02-1.46 | 0.03 |
| Never | reference | |
| Age started smoking | 0.95, 0.92-0.99 | 0.01 |
| Pack years | 1.02, 1.01-1.03 | <0.001 |
| Heavy smoker | 1.59, 1.13-2.25 | 0.01 |
| Moderate smoker | 1.47, 1.15-1.87 | 0.02 |
| Light smoker | 1.20, 1.00-1.45 | 0.05 |
| Non-smoker | reference | |
| Passive smoking | ||
| Participant’s father smoker | 1.12, 0.96-1.31 | 0.15 |
| Participant’s partner smoker | 1.14, 0.98-1.32 | 0.09 |
information assessed at baseline time point
Analyses were adjusted for factors associated with both in-utero exposure and age at menopause (pack years, age at OC use start, age at first pregnancy and participant’s age). Pack years was removed from the adjusted model when other characteristics associated with active smoking of the participants were being analyzed to avoid double correction for smoking factors. Please see supplementary table II for the comparison between crude and adjusted Hr for menopause for all factors. There was a trend observed for the risk of menopause, with heavy smokers having the highest risk (HR 1.59 95%CI 1.13-2.25; adjusted HR 1.64 95%CI 1.16-2.33), followed by moderate smokers (HR 1.47 95%CI 1.15-1.87; adjusted HR 1.32 95%CI 1.02-1.70) and at last light smokers (HR 1.20 95%CI 1.00-1.45; adjusted 1.19 95%CI 0.98-1.45).
Table III shows crude and adjusted HR for participants who were in-utero exposure, ever smokers and, for stratified groups of in-utero exposure and ever smoking combined. In-utero smoke exposure was not associated with earlier menopause. There an interaction between in-utero smoke exposure and pack years of participants (p=0.02). The stratified analysis confirmed a stronger effect of ever smoking and in-utero exposure on age at menopause, i.e. smokers who had been exposed in-utero had higher risk when compared to smokers or smokers who were not exposed. .
Table III.
Crude and adjusted HR for participants who were in-utero exposure, active smokers and, for stratified groups of in-utero exposure and active smoking combined.
| Crude HR, 95% CI | p-value | Adjusted HR, 95% CI | p-value | |
| In-utero exposure (n=2852) | 0.98, 0.81-1.19 | 0.86 | 0.98, 0.80-1.19b | 0.81 |
| Evera smoker (n=2831) | 1.31, 1.10-1.56 | 0.002 | 1.30, 1.08-1.56c | 0.006 |
| Stratified on groups | ||||
| In-utero exposed and ever smoker (n=199) | 1.50, 1.09-2.06 | 0.01 | 1.41, 1.01-1.95c | 0.04 |
| In-utero exposed and non-smoker (n=373) | 0.91, 0.72-1.14 | 0.40 | 0.92, 0.72-1.18c | 0.51 |
| Not exposed and ever smoker (n=516) | 1.23, 1.01-1.50 | 0.04 | 1.24, 1.00-1.53c | 0.05 |
| Not exposed and non-smoker (n=1743) | reference | reference |
ever smokers include the current and previous smokers.
adjusted for age of participants, age at OC start, age at 1st pregnancy and pack years.
adjusted for age of participants, age at OC start and age at 1st pregnancy
Sensitivity analysis with participants with available age at menopause only did not change the results (HR 0.98, 0.75-1.29; adjusted HR 0.97, 0.73-1.29, see supplementary table I).
Participants were not included in the analyses for two reasons: missing data on in-utero exposure (n=663) and excluded participants due to other reasons (n=617). There were no statistically significant differences regarding smoking status between participants who had available(26.2%, 746/2852) and those who had missing data (28.2%, 187/663) regarding in-utero exposure (p-value= 0.49). For the group who was excluded for other reasons, smoking status was slightly higher (31.9%, 197/617 versus 26.5%, 933/3515, p-value =0.004) as well as in-utero exposure (24.6%, 122/495 versus 20.2%, 577/2852, p-value= 0.03). Analysis including these participants (n=3347) showed no substantial difference (OR 0.91, 95%CI 0.76-1.08, adjusted OR 0.86, 95% CI 0.70-1.04) compared to the analysis excluding this group (table III).
Discussion
Results from the present study indicate that in-utero smoke exposure is not associated with earlier age at menopause. There was an interaction between pack years of participants and in-utero smoke exposure, i.e. the effect of in-utero smoke exposure was modified by the smoking habits of the participants themselves.
Strengths of this study are the large number of participants and the substantial proportion of postmenopausal women. Moreover, the 18-year follow up, the availability of repeated questionnaires and information regarding various factors relevant for the risk of menopause are strong points. This allowed the calculation of pack years for participants who were ever smokers, so that the confounding effect of smoking of participants could be analyzed in a dose-response manner.
Although there is no previous data available reporting the accuracy of in-utero smoke exposure based on questionnaires, in this data participants who reported being in-utero smoke exposed had a lower birth weight and the agreement between questionnaire from two different time points were satisfactory. This indicates low misclassification and recall bias.
Limitations included no information about the number of cigarettes smoked by the mothers while they were pregnant with the participants, no information about trimester of exposure and, incomplete information on age at menopause. Higher levels of in-utero smoke exposure could have an effect on age at menopause, similar to the dose effect relationship observed for the smoking habits during adulthood. Given the time period during which these pregnancies occurred, i.e. around the mid-1960’s, we assumed mothers of the participants would have smoked during the entire pregnancy. Smoking was widely accepted in this period17 and the effects of smoking during pregnancy were not widely known18.
In the current study, we had to estimate age at menopause by subtracting one year of the questionnaire’s age at completion for 51.7% (359/695) of participants who reported they were postmenopausal. This may have resulted in shifting age at menopause to a later age for women who went into menopause more than one year before completion of the questionnaire. Nevertheless, analysis including only participants with available age at menopause showed a similar effect compared to the analysis including women whose age at menopause was estimated.
The long follow-up period resulted in a dropout rate of 38% (14541-9028/14541)and there was a response rate of 46.2% (4175/9028), when considering participants who were still active in the cohort. Therefore, our results are based on 20% (2852/15541) of the original data, from which results cannot be extended and as consequence we cannot exclude the risk of selection bias19. Participants in this study were pregnant when they were recruited. Therefore, results cannot be readily extended to nulliparous women.
Our hypothesis, that in-utero exposed women have higher hazards of menopause, was not confirmed by our results. Previous studies have shown results both similar and dissimilar to ours, and the method of analysis may have played a role in this. Results similar to ours were observed in a large dataset (n=22165), with 30% postmenopausal participants and 36% in-utero exposure20. The authors censored participants who were postmenopausal due to surgery, just as we have done in our analysis, and found that in-utero exposure was not associated with earlier menopause (HR 1.02 95%CI 0.97-1.7). Another study (n=4025) with 37.0% postmenopausal participants and 14.8% in-utero exposure, has shown an effect on age at menopause (HR 1.21 95% CI 1.02-1.43)21. In this study, in-utero exposed women who did not smoke had higher hazards of menopause (1.38 95%CI 1.10-1.74) compared to the smokers (1.03 95%CI 0.81-1.31). However, this study was performed in a cohort in which 80% of the women were prenatally exposed to diethylstilbestrol (DES). DES is known for its reductive influence on development competence of primordial follicles22. Therefore, these results might be limited to this specific prenatal DES-exposed population.
In the study of Tawfik and colleagues, 1001 women were analyzed, of whom 3.8% were postmenopausal and 40% were exposure in-utero23. The authors used logistic regression instead of Cox regression, differently from the other studies previously described above. Smoking participants who were exposed in-utero had the highest odds of menopause (OR 3.4, 95% CI 1.1-10.3), followed by smokers who were not exposed in (OR 2.8 95% CI 0.9-9.0). In their analyses, the authors controlled for age of the participants and, since censoring was not an option with the logistic regression model, they excluded women who used HT or had ovarian or uterine surgery. When we repeated their analyses in our data, we found similar results to theirs which were in the same direction and with higher estimates (OR 2.23, 95% CI 1.25-3.97; OR 1.74, 95% CI 1.21-2.49, respectively) compared to the analysis with Cox regression including HT and participants who had surgery. With the exclusion of these women from our data, the estimates with Cox regression were also slightly increased (HR 1.55, 95%CI 1.01-2.36 and HR 1.37, 95%CI 1.06-1.77). Our conclusion is based on the analysis with Cox regression censoring participants who used HT or who had surgery since this is the most appropriate method to avoid bias in Cox Regression analysis24. When censoring, the analysis takes into consideration that up to the point that participants started using HT or had surgery, participants were still premenopausal and that is important information to include in the analysis. We consider that excluding these women introduces bias, resulting in overestimation of the risk.
Despite the negative effect that smoking has on age at menopause for smokers themselves, the same effect was not observed for women who were only in-utero exposed. One explanation for this may be the strongly programmed mechanism that determines age at menopause. This evidence comes from studies in which women who had unilateral oophorectomy, despite losing half of their ovarian reserve, reached menopause only one year earlier25,26. Some studies suggest compensatory growth of the remaining ovary27 This indicates that there could be other determinants for the onset of menopause additionally to the size of the follicle pool. Van Asselt and colleagues argue that the capacity of remaining follicles to produce estrogens can be relevant as well for onset of menopause28. Another explanation might be the existence of counterbalancing protective mechanisms such as recruitment in wave-like fashion of less oocytes per cycles29 or longer cycle lengths so that the total number of ovulation in a lifetime is shortened30, supported by studies that showed an earlier age at menopause for mothers of dizygotic twins compared to control mothers31,32. Germline stem cells in adult human ovaries, which could be activated postnatally under specific circumstances33 could be another possibility but robust human data is necessary to support this hypothesis34,35.
Conclusions
Our results indicate that in-utero smoke exposure is not associated with earlier menopause, though smoking of participants is. There was an interaction observed between in-utero smoke exposure and pack years of participants, i.e., the effect of in-utero smoke exposure was modified by the smoking habits of the participants themselves. Despite the effect of adult active smoking on age at menopause and the significant reduction of the follicle pool associated with in-utero smoke exposure shown in experimental studies, our epidemiological study does not support similar mechanisms for in-utero exposed women who are not smokers themselves. Counterbalancing protective mechanisms may play an important role in order to maintain the predetermined age at menopause.
Supplementary Material
Footnote:
a. ever smokers include the current and previous smokers. b. adjusted for age of participants, age at OC start, age at 1st pregnancy and pack years. c. adjusted for age of participants, age at OC start and age at 1st pregnancy.
Footnote:
a. information assessed at baseline time point b. adjusted for age of participants, age at OC start, age at 1st pregnancy and pack years. c. adjusted for age of participants, age at OC start and age at 1st pregnancy.
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and will serve as guarantors for the contents of this paper. This research was specifically funded by the Department of Obstetrics and Gynecology, UMCG.
Financial support: Department of Obstetrics and Gynecology ,section of Reproductive Medicine, University Medical Center Groningen.
Footnotes
Conflict of interest: Outside the submitted work, the Fertility Center of the UMCG received an unrestricted educational grant from Ferring pharmaceuticals BV, The Netherlands.
References
- 1.MJ F, RG G. A model conforming the decline in follicle numbers to the age of menopause in women. Hum Reprod. 1996 Jul;11(7):1484–6. doi: 10.1093/oxfordjournals.humrep.a019422. (0268-1161 (Print); 0268-1161 (Linking)) [DOI] [PubMed] [Google Scholar]
- 2.Coxworth JE, Hawkes K. Ovarian follicle loss in humans and mice: lessons from statistical model comparison. Hum Reprod. 2010;25(7):1796–1805. doi: 10.1093/humrep/deq136. [DOI] [PubMed] [Google Scholar]
- 3.Mamsen LS, Lutterodt MC, Andersen EW, Byskov AG, Andersen CY. Germ cell numbers in human embryonic and fetal gonads during the first two trimesters of pregnancy: analysis of six published studies. Hum Reprod. 2011;26(8):2140–2145. doi: 10.1093/humrep/der149. [DOI] [PubMed] [Google Scholar]
- 4.Depmann M, Faddy MJ, van der Schouw YT, et al. The Relationship Between Variation in Size of the Primordial Follicle Pool and Age at Natural Menopause. J Clin Endocrinol Metab. 2015;100(6):E845–51. doi: 10.1210/jc.2015-1298. [DOI] [PubMed] [Google Scholar]
- 5.Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5(1):e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson MC, Guo M, Fauser BC, Macklon NS. Environmental and developmental origins of ovarian reserve. Hum Reprod Update. 2014;20(3):353–369. doi: 10.1093/humupd/dmt057. [DOI] [PubMed] [Google Scholar]
- 7.Parente RC, Faerstein E, Celeste RK, Werneck GL. The relationship between smoking and age at the menopause: A systematic review. Maturitas. 2008;61(4):287–298. doi: 10.1016/j.maturitas.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Sun L, Tan L, Yang F, et al. Meta-analysis suggests that smoking is associated with an increased risk of early natural menopause. Menopause. 2012;19(2):126–132. doi: 10.1097/gme.0b013e318224f9ac. [DOI] [PubMed] [Google Scholar]
- 9.Bussmann UA, Baranao JL. Regulation of aryl hydrocarbon receptor expression in rat granulosa cells. Biol Reprod. 2006;75(3):360–369. doi: 10.1095/biolreprod.106.053017. doi:biolreprod.106.053017 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Gershon E, Plaks V, Dekel N. Gap junctions in the ovary: expression, localization and function. Mol Cell Endocrinol. 2008;282(1–2):18–25. doi: 10.1016/j.mce.2007.11.001. doi:S0303-7207(07)00411-X [pii] [DOI] [PubMed] [Google Scholar]
- 11.Dechanet C, Anahory T, Daude JCM, et al. Effects of cigarette smoking on reproduction. Hum Reprod Update. 2011;17(1):76–95. doi: 10.1093/humupd/dmq033. [DOI] [PubMed] [Google Scholar]
- 12.Camlin NJ, Sobinoff AP, Sutherland JM, et al. Maternal Smoke Exposure Impairs the Long-Term Fertility of Female Offspring in a Murine Model. Biol Reprod. 2016;94(2):39. doi: 10.1095/biolreprod.115.135848. [DOI] [PubMed] [Google Scholar]
- 13.Lutterodt MC, Sorensen KP, Larsen KB, Skouby SO, Andersen CY, Byskov AG. The number of oogonia and somatic cells in the human female embryo and fetus in relation to whether or not exposed to maternal cigarette smoking. Hum Reprod. 2009;24(10):2558–2566. doi: 10.1093/humrep/dep226. [DOI] [PubMed] [Google Scholar]
- 14.Boyd A, Golding J, Macleod J, et al. Cohort Profile: the “children of the 90s”--the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42(1):111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golding J. Children of the nineties. A longitudinal study of pregnancy and childhood based on the population of Avon (ALSPAC) West Engl Med J. 1990;105(3):80–82. [PMC free article] [PubMed] [Google Scholar]
- 16.Golding J, Pembrey M, Jones R, Team AS. ALSPAC--the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15(1):74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 17.Pierce JP, Lee L, Gilpin EA. Smoking initiation by adolescent girls, 1944 through 1988. An association with targeted advertising. Jama. 1994;271(8):608–611. [PubMed] [Google Scholar]
- 18.Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J. 1950;2(4682):739–748. doi: 10.1136/bmj.2.4682.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiner AZ, D’Aloisio AA, DeRoo LA, Sandler DP, Baird DD. Association of intrauterine and early-life exposures with age at menopause in the Sister Study. Am J Epidemiol. 2010;172(2):140–148. doi: 10.1093/aje/kwq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strohsnitter WC, Hatch EE, Hyer M, et al. The association between in utero cigarette smoke exposure and age at menopause. Am J Epidemiol. 2008;167(6):727–733. doi: 10.1093/aje/kwm351. [DOI] [PubMed] [Google Scholar]
- 22.Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142(15):2554–2563. doi: 10.1242/dev.125211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tawfik H, Kline J, Jacobson J, et al. Life course exposure to smoke and early menopause and menopausal transition. Menopause. 2015;22(10):1076–1083. doi: 10.1097/GME.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Walraven C, Hawken S. Competing risk bias in Kaplan-Meier risk estimates can be corrected. J Clin Epidemiol. 2016;70:101–105. doi: 10.1016/j.jclinepi.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Bjelland EK, Wilkosz P, Tanbo TG, Eskild A. Is unilateral oophorectomy associated with age at menopause? A population study (the HUNT2 Survey) Hum Reprod. 2014;29(4):835–841. doi: 10.1093/humrep/deu026. [DOI] [PubMed] [Google Scholar]
- 26.Yasui T, Hayashi K, Mizunuma H, et al. Factors associated with premature ovarian failure, early menopause and earlier onset of menopause in Japanese women. Maturitas. 2012;72(3):249–255. doi: 10.1016/j.maturitas.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Roth JJ, Jones RE. A single ovary of Anolis carolinensis responds more to exogenous gonadotropin if the contralateral ovary is absent. Gen Comp Endocrinol. 1992;85(3):486–492. doi: 10.1016/0016-6480(92)90093-Y. [DOI] [PubMed] [Google Scholar]
- 28.van Asselt KM, Kok HS, van Der Schouw YT, et al. Current smoking at menopause rather than duration determines the onset of natural menopause. Epidemiology. 2004;15(5):634–639. doi: 10.1097/01.ede.0000134868.53468.b7. 00001648-200409000-00022 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Baerwald AR, Adams GP, Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril. 2003;80(1):116–122. doi: 10.1016/S0015-0282(03)00544-2. [DOI] [PubMed] [Google Scholar]
- 30.Cramer DW, Barbieri RL, Xu H, Reichardt JK. Determinants of basal follicle-stimulating hormone levels in premenopausal women. J Clin Endocrinol Metab. 1994;79(4):1105–1109. doi: 10.1210/jcem.79.4.7962282. [DOI] [PubMed] [Google Scholar]
- 31.Martin NG, Healey SC, Pangan TS, Heath AC, Turner G. Do mothers of dizygotic twins have earlier menopause? A role for fragile X? Am J Med Genet. 1997;69(1):114–116. doi: 10.1002/(SICI)1096-8628(19970303)69:1<114::AID-AJMG23>3.0.CO;2-R. [pii] [DOI] [PubMed] [Google Scholar]
- 32.Van der Stroom EM, Konig TE, Vink JM, Boomsma DI, Lambalk CB. Ovarian reserve and anti-Mullerian hormone (AMH) in mothers of dizygotic twins. Twin Res Hum Genet. 2013;16(2):634–638. doi: 10.1017/thg.2013.4. [DOI] [PubMed] [Google Scholar]
- 33.Tilly JL, Telfer EE. Purification of germline stem cells from adult mammalian ovaries: a step closer towards control of the female biological clock? Mol Hum Reprod. 2009;15(7):393–398. doi: 10.1093/molehr/gap036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. doi: 10.1097/00006254-200407000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Hikabe O, Hamazaki N, Nagamatsu G, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539(7628):299–303. doi: 10.1038/nature20104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Footnote:
a. ever smokers include the current and previous smokers. b. adjusted for age of participants, age at OC start, age at 1st pregnancy and pack years. c. adjusted for age of participants, age at OC start and age at 1st pregnancy.
Footnote:
a. information assessed at baseline time point b. adjusted for age of participants, age at OC start, age at 1st pregnancy and pack years. c. adjusted for age of participants, age at OC start and age at 1st pregnancy.