Abstract
Background
Desmoplastic melanoma (DM) is frequently misdiagnosed clinically and often associated with melanoma in situ (MIS).
Objective
To improve the detection of DM using dermoscopy and reflectance confocal microscopy (RCM).
Methods
A descriptive analysis of DM dermoscopy features and a case-control study within a melanoma population for RCM feature evaluation, performed blindly, using data obtained between 2005 and 2015. After retrospectively identifying all DM cases with RCM data over the study period (n=16), a control group of non-DM melanoma patients with RCM data, in a ratio of at least 3:1, was selected. The control group was matched by age and primary tumor site location, divided into non-DM invasive melanomas (n=27) and MIS (n=27). Invasive melanomas were selected according to the melanoma subtypes associated with the DM cases. The main outcomes were the frequency of melanoma-specific features on dermoscopy for DM; and the odds ratios of RCM features to distinguish DM from MIS and/or other invasive melanomas; or MIS from the combined invasive melanoma group.
Results
At least 1 of the 14 melanoma-specific features evaluated on dermoscopy were found in 100% of DMs (n=15 DM with dermoscopy). Known RCM melanoma predictors were commonly found in the DMs, such as pagetoid cells (100%) and cell atypia (100%). The RCM feature of spindle cells in the superficial dermis was more common in DM compared with the entire melanoma control group (OR 3.82, 95% CI 1.01–14.90), and particularly compared to MIS (OR 5.48, 95% CI 1.11–32.36). Nucleated cells in the dermis and the RCM correlate of dermal inflammation were also significant RCM features favouring DM over MIS, as well as invasive melanoma over MIS.
Conclusion
Dermoscopy and RCM may be useful tools for the identification of DM. Certain RCM features may help distinguish DM from MIS and other invasive melanomas. Larger studies are warranted.
Introduction
Desmoplastic melanoma (DM) is an uncommon type of invasive melanoma, representing less than 5% of all melanomas.1,2 DM is characterised histologically by sparsely distributed spindle cells in a fibrocollagenous stroma.3,4 In approximately three-quarters of DMs, there is an overlying melanoma in situ (MIS) (usually of lentigo maligna [LM] type) or atypical melanocytic hyperplasia.2,5 It is important to recognize DM from other types of melanoma for two reasons. Firstly, DM has a different clinical behavior compared to other types of melanoma, which may influence management decisions.3,6 This includes a higher local recurrence rate, and less propensity to metastasize to the regional lymph nodes in DM.3,6 These behaviors are influenced by the proportion of the tumor that is desmoplastic.3,4
Secondly, DM is commonly associated with large MIS, and also commonly occurs in the head and neck region,2,5 where cosmesis is a concern. Should partial biopsy be undertaken in such circumstances, site selection must be carefully determined to sample from the region thought most likely to have an invasive melanoma component, so that a DM or other type of invasive melanoma is not missed. This is especially relevant in the context of non-invasive treatments for MIS like radiotherapy or imiquimod, which would not be appropriate on their own for primary invasive melanoma.
DM may be difficult to clinically diagnose as they are frequently mistaken for benign lesions or other types of skin cancer.5 Dermoscopy may provide clues toward a diagnosis of DM, such as atypical vascular structures and peppering.5,7
In order to facilitate accurate diagnosis of DM, we sought to investigate the features of DM with dermoscopy and reflectance confocal microscopy (RCM) and compare them with MIS and other types of invasive melanoma. Our first hypothesis was that dermoscopy could be used to help identify DM (simulating the clinical scenario where dermoscopy is used as a screening tool - so that a biopsy for lesions suspicious of DM then becomes warranted). The second hypothesis was that RCM could be useful to distinguish DM (with or without an in situ component) from MIS, and/or non-DM invasive melanoma (simulating the scenario of when RCM is used to help determine where to biopsy from a suspicious lesion). Our final hypothesis was that, should RCM not be sufficient in clinically distinguishing DM from other melanoma subtypes, RCM could at least be used to determine if the melanoma (irrespective of subtype) is in situ or invasive (to enable a targeted biopsy to the area most likely to be invasive, or prompt complete excision of the lesion).
Methods
Study design and patient recruitment
This was a case-control study taken from data accrued between 2005 to 2015. DM cases were identified from 3 different tertiary centres for melanoma diagnosis that included Australia (Melanoma Institute Australia and Sydney Melanoma Diagnostic Centre, Sydney), Spain (Melanoma Unit, Dermatology Department, Hospital Clinic of Barcelona, Barcelona) and Italy (Department of Dermatology, University of Modena and Reggio Emilia, Modena).
The study was covered under local governance board and ethics committee approvals from the Royal Prince Alfred Hospital zone of Sydney local area health district (X15–0392), and Hospital Clínic de Barcelona (HCB/2014/0023, HCB/2015/0146).
A ratio of at least 3:1 control cases for each DM was sought. Control cases were other types of melanoma found from a RCM database in Sydney, so that pathology records for these cases could be directly accessed by the first author. The controls were matched to the age (by average of +/− 5 years), and site location frequency (+/− 10%) of the DM group. The control group was weighted equally to contain non-DM MIS, and non-DM invasive melanomas. This weighting represented an awareness that DM is commonly associated with MIS2, and ensured that our hypotheses could be tested. The non-DM invasive melanomas were selected in a greater than 1:1 proportion by the subtypes of invasive melanoma associated with the DM cases found.
Inclusion and exclusion criterion
The main inclusion criterion was RCM images of a histopathologically confirmed melanoma. Clinical and dermatoscopic images, along with representative histopathological images from all DM cases were also sought. The exclusion criteria were any melanomas without histopathology after RCM was taken. All patients gave verbal consent for the imaging completed in the study and received no compensation for their participation. RCM imaging in all centres was performed with Vivascope® 1500 or Vivascope® 3000 machines (Caliber Imaging and Diagnostics Inc, NY, U.S.A.).
Study variables
Patient and lesion details were recorded as noted in Table 1. DM cases had dermoscopy characteristics recorded according to the study by Jaimes et al.5 (P.G.). Histopathology images of the DM cases underwent central review by a histopathology team (R.S., A.S.), with the features noted described in Table 1 and Table 2, along with assessment for neurotropia (present or absent) and percentage desmoplasia.
Table 1.
Study demographics and histopathological characteristics
| Feature | No. DM (%) (n=16) |
No. In situ (%) (n=27) |
No. Non-DM invasive (%) (n=27) |
|
|---|---|---|---|---|
| Mean age in years [SD] | 68 [16.9] | 69 [14] | 63 [18.1] | |
| Gender | ||||
| Male | 12 (75) | 14 (52) | 13 (48) | |
| Female | 4 (25) | 13 (48) | 14 (52) | |
| Location | ||||
| Head/neck | 10 (63) | 19 (70) | 14 (52) | |
| Trunk | 2 (13) | 4 (15) | 3 (11) | |
| Limb | 3 (19) | 4 (15) | 7 (26) | |
| Acral | 1 (5) | 0 | 3 (11) | |
| Mean Breslow thickness in mm [SD] | 3.3 [2.36]a | N/a | 0.7 [0.56] | |
| DM Type, n=14b | Purec | Mixedc | ||
| 6/14 (43) | 8/14 (57) | |||
| Main or associated invasive melanoma typeb | ||||
| SSM | 0 | 2/14 (14) | 0 | 7 (26) |
| LMM | 0 | 2/14 (14) | 0 | 11 (41) |
| SSM/LMM | 0 | 0 | 0 | 2 (7) |
| ALM | 0 | 1/14 (7) | 0 | 3 (11) |
| Nodular/naevoid | 0 | 3/14 (21) | 0 | 4 (15) |
| In situ component (if present)b | ||||
| LM | 3/14 (21) | 3/14 (21) | 24 (89) | NR |
| In situ SSM | 1/14 (7) | 2/14 (14) | 2 (7) | NR |
| In situ ALM | 0 | 1/14 (7) | 0 | NR |
| In situ NS | 0 | 0 | 1 (4) | NR |
| No in situ | 2/14 (14) | 2/14 (14) | 0 | NR |
| Predominant cell typeb | ||||
| Spindle | 6/14 (43) | 6/14 (43) | N/a | 3 (11) |
| Spindle and Naevoid | 0 | 1/14 (7) | N/a | 1 (4) |
| Spindle and Epitheloid | 0 | 1/14 (7) | N/a | 1 (4) |
| Epitheloid | 0 | 0 | N/a | 12 (44) |
| Naevoid | 0 | 0 | N/a | 2 (7) |
| Epitheliod and Naevoid | 0 | 0 | N/a | 1 (4) |
| NS | 0 | 0 | N/a | 7 (26) |
3 cases missing Breslow depth data.
2 DM cases did not have histopathological slides available for central review.
Pure DM ≥90% desmoplasia, mixed desmoplastic melanoma <90% desmoplasia.
DM, desmoplastic melanoma; SSM, superficial spreading melanoma; LMM, lentigo maligna melanoma; ALM, acral lentiginous melanoma; LM, lentigo maligna; N/a, non applicable; NR, not recorded; NS, Non-specified.
Table 2.
Selected histological-RCM correlations in DM
| Feature | No. (%)
|
|
|---|---|---|
| Histology (n=14) |
RCM (n=14) |
|
| Spindle cells in superficial dermis (or within 0.3mm of surface on histopathology) | ||
| Present | 13 (93) | 7 (50) |
| Absent | 1 (7) | 7 (50) |
| Dermal Inflammation | ||
| Absent | 2 (14) | 7 (50) |
| Mild | 9 (64) | 6 (43) |
| Diffuse | 3 (21) | 1 (7) |
| Present (mild or diffuse) | 12 (86) | 7 (5) |
| Pagetoid spread | ||
| Absent | 6 (43) | 1 (7) |
| 1= 1–2 atypical cells in 5 fields | 7 (50) | 4 (29) |
| 2= ≥3 atypical cells in 5 fields | 1 (7) | 9 (64) |
| Present (1 or 2) | 8 (57) | 13 (93) |
| Density of junctional melanocytes | ||
| Absent | 1 (7) | 2 (14) |
| 1= focal or scattered | 7 (50) | 6 (43) |
| 2= touching each other | 6 (43) | 6 (43) |
| Present (1 or 2) | 13 (93) | 12 (86) |
| Plump bright cells in superficial dermis (or pigment incontinence for histology) | ||
| Present | 10 (71) | 12 (86) |
| Absent | 4 (29) | 2 (14) |
RCM, reflectance confocal microscopy.
RCM characteristics evaluated included those relevant to melanoma defined from previous observations8–11, and other parameters described in Table 2. Dermal inflammation on RCM was defined as “diffuse single or aggregated small (<12μm) round-to-polygonal mildly refractive cells at the dermal level”.12 RCM features were analysed blinded to dermoscopy and histopathology (N.G.M.). The RCM score for melanoma diagnosis8 was calculated for all lesions in the study, and the LM score13 was calculated for the MISs.
Data analysis
Statistical analysis was performed using IBM® SPSS® software (IBM SPSS statistics for Windows, release 22.0.0, IBM Corp, Armonk, NY, USA), and SAS software (version 9.4, © 2002–2012, SAS Institute Inc., Cary, NC, USA).
To address our first hypothesis, the frequency of dermatoscopic melanocytic and melanoma-specific features of the DM group were evaluated, and compared to those recorded in the DM study by Jaimes et al.5
To address our second hypothesis, exact logistic regression analyses were conducted to examine the association of RCM features to DM compared to the melanoma controls (DM versus all melanoma controls, DM versus non-DM MIS, and DM versus non-DM invasive melanoma). Exact logistic regression was used due to the small data set. The same method was also used to compare the association of RCM features between MIS and the combined DM and non-DM invasive group of melanomas.
Descriptive and Cohen ĸ statistics were calculated for RCM-histological agreement between analogous variables assessed for the DM cases. Cohen ĸ statistics were also used to examine the correlation between spindle cells in the superficial dermis on RCM and spindle cells as the predominant cell type found from the invasive melanomas.
P values were calculated as two-tailed, and a P value of <0.05 was regarded as significant.
Results
The study demographics and summary histopathological characteristics found for the DM, MIS and non-DM invasive melanomas are shown in Table 1. The range of Breslow depths for the DM patients with this data available (n=13) was 1.1–10mm.
Dermoscopy
For the 15 DM that had dermoscopy available (dermoscopy data was missing for 1 patient), 1 had one colour only, 2 had two colours, and 12 had three or more colours (Fig. 1). The colours included pink/red (14 of 15 [93%]), light brown (12 of 15 [80%]), dark brown (11 of 15 [73%]), white (11 of 15 [73%]), black (9 of 15 [60%]) and blue (2 of 15 [13%]). Of these 15 DM, 1 DM was completely flat, 12 were flat with a raised component (of which 8 were papules, 1 was nodular, and 3 were papular and nodular) and 2 were raised lesions only (both nodules). All 15 cases demonstrated ill-defined borders. Table 3 shows the frequency of DM dermoscopic features in our series compared with those from the Jaimes et al.5 study, which is the largest dermoscopy study on DM that the authors are aware of.
Figure 1.
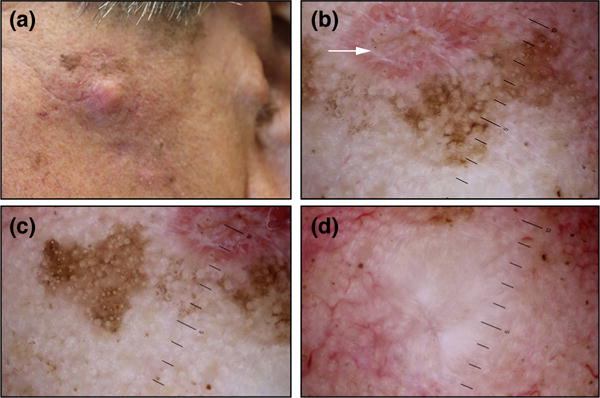
A man in his 70s with a pale nodule and an indurated periphery with brown, pink and red colouration on the left cheek, that revealed a desmoplastic melanoma on wide excision (a). Dermoscopy demonstrates a healing scar from an earlier shave biopsy (that revealed lentigo maligna, white arrow), along with atypical vascular structures, peppering, crystalline structures, annular granular pattern, pseudonetwork, follicular obliteration, and regression areas (b-d).
Table 3.
Dermoscopy characteristics of the DM compared to the largest study to date with dermoscopy in DM by Jaimes et al.(5)
| Dermoscopy Characteristic | No. DM (%) (n=15) | Jaimes et al.(5) No. DM (%) (n=37) |
|---|---|---|
| Melanocytic structures present | 9 (60) | 16 (43) |
| Globules (atypical) | 3 (20) | 7 (44) |
| Pigment network (typical or atypical) | 7 (47) | 6 (38) |
| Negative network | 0 (0) | 1 (6) |
| Homogenous blue pigmentation | 0 (0) | 0 (0) |
| Streaks | 1 (7) | 0 (0) |
| Pseudonetwork (facial skin) | 6 (40) | 4 (25) |
| Melanoma-specific structures present | 15 (100) | 37 (100) |
| Atypical vascular structures | 13 (87) | 30 (81) |
| Peppering | 4 (27) | 12 (32) |
| Crystalline structures | 4/5 (80)a | 12/15 (80)a |
| Annular-granular pattern | 6 (40) | 9 (24) |
| Blue-white veil | 2 (13) | 7 (19) |
| Atypical globules | 3 (20) | 7 (19) |
| Atypical network | 5 (33) | 5 (14) |
| Scarlike areas | 10 (67) | 3 (8) |
| Off-center blotch | 9 (60) | 3 (8) |
| Peripheral tan structureless areas | 12 (80) | 1 (3) |
| Negative network | 0 (0) | 1 (3) |
| Streaks | 1 (7) | 0 (0) |
| Polygonal lines | 1 (7) | 4 (11) |
| Follicular obliteration | 4 (27) | 1 (3) |
| Vascular structures present | 13 (87) | 30 (81) |
| Dotted vessels | 2 (13) | 6 (38) |
| Serpentine vessels (linear irregular) | 9 (60) | 4 (11) |
| Coiled vessels | 2 (13) | 1 (3) |
| Vascular blush/milky-red areas | 9 (60) | 20 (67) |
| Polymorphous vessels (>2 types) present | 9 (60) | 13 (43) |
Only assessed from cases with polarized dermoscopy.
RCM
Table 4 shows the frequency of RCM features recorded in the study. Strong RCM melanoma predictors8,10 were commonly found in the DM population (pagetoid cells 100%, cell atypia 75%, nucleated cells in the dermis 75%). Only 2 melanomas scored <3 on the RCM score (1 DM, and 1 MIS – both had scores of 2), giving a 97% sensitivity for melanoma diagnosis with a RCM score of ≥3. For the LM score, 4 MISs scored <2, giving a sensitivity of 85% for MIS diagnosis in our series at this cut-off. Table 5 shows the odds ratios (OR) for the RCM variables, comparing DM to the melanoma controls, and comparing the combined DM and other invasive melanoma group to the MIS group. The RCM feature of spindle cells in the superficial dermis was more common in DM compared with the entire melanoma control group (OR 3.82, 95% CI 1.01–14.90), and particularly compared to MIS (OR 5.48, 95% CI 1.11–32.36). Nucleated cells in the dermis and the RCM correlate of dermal inflammation were also significant RCM features more commonly found in DM compared to MIS (OR 5.73, 95% CI 1.27–31.80; and OR 1.94, 95% CI 0.36–3.73 respectively), as well as invasive melanoma compared to MIS (OR 4.51, 95% CI 1.47–14.80; and OR 5.87, 95% CI 1.62–27.34 respectively). Round pagetoid cells on RCM were less likely to be present in DM compared to all other invasive melanomas (OR 0.09, 95% CI 0–0.58).
Table 4.
Frequency of RCM features in DM, MIS, and non-desmoplastic invasive melanomas
| RCM Feature | No. (%)
|
||
|---|---|---|---|
| DM (n=16) | Non-DM in situ (n=27) | Non-DM invasive (n=27) | |
| Regular HC pattern | 11 (69) | 18 (67) | 13 (48) |
| Atypical HC | 10 (63) | 14 (52) | 16 (59) |
| Broadened HC | 6 (38) | 10 (37) | 13 (48) |
| Diamond-shaped HC | 0 | 0 | 0 |
| Cobblestone pattern | 3 (19) | 4 (15) | 1 (4) |
| Atypical cobblestone pattern | 0 | 3 (11) | 3 (11) |
| Atypical cobblestone pattern with small nucleated cells | 0 | 2 (7) | 2 (7) |
| Epidermal disarray | 6 (38) | 11 (41) | 5 (19) |
| HC atypical and disarray | 5 (31) | 5 (19) | 3 (11) |
| Pagetoid cells | 16 (100) | 27 (100) | 27 (100) |
| Widespread pagetoid infiltration | 10 (63) | 14 (52) | 12 (44) |
| Round pagetoid cells | 12 (75) | 22 (81) | 27 (100) |
| Dendritic pagetoid cells | 16 (100) | 25 (93) | 23 (85) |
| Pagetoid cells around follicular opening | 2 (13) | 6 (22) | 4 (15) |
| Edged papillae | 7 (44) | 13 (48) | 8 (30) |
| Non-edged papillae | 8 (50) | 19 (70) | 18 (67) |
| Large interpapillary space | 0 | 0 | 0 |
| Polycyclic papillae | 2 (13) | 7 (26) | 5 (19) |
| Papillae enlarged and polycyclic | 1 (6) | 3 (11) | 3 (11) |
| Non-visible papillae | 5 (31) | 7 (26) | 9 (33) |
| Cell atypia | 16 (100) | 27 (100) | 27 (100) |
| Marked atypia | 8 (50) | 14 (52) | 14 (52) |
| Sheet of cells | 4 (25) | 6 (22) | 6 (22) |
| Junctional nest | 4 (25) | 7 (26) | 6 (22) |
| Junctional clusters | 4 (25) | 6 (22) | 6 (22) |
| Junctional thickening | 1 (6) | 1 (4) | 2 (7) |
| Nests | 2 (13) | 6 (22) | 10 (37) |
| Dense nest | 0 | 3 (11) | 2 (7) |
| Dishomogenous nest | 2 (13) | 4 (15) | 9 (33) |
| Sparse nest | 1 (6) | 4 (15) | 6 (22) |
| Cerebriform nest | 0 | 0 | 3 (11) |
| Plump bright cells | 14 (88) | 16 (59) | 18 (67) |
| Plump bright cells in large aggregation within papillary dermis | 4 (25) | 5 (19) | 5 (19) |
| Vessels: visible | 5 (31) | 5 (19) | 8 (30) |
| Vertical vessel within the papillae | 1 (6) | 0 | 1 (4) |
| Horizontal vessel | 4 (25) | 5 (19) | 5 (19) |
| Linear telangiectasia-like horizontal vessel | 1 (6) | 2 (7) | 3 (11) |
| Convoluted glomerular-like vessel | 1 (6) | 1 (4) | 3 (11) |
| Hyporeflective pagetoid cells | 1 (6) | 4 (15) | 2 (7) |
| Spindle cells in superficial dermis | 8 (50) | 4 (15) | 7 (26) |
| Nucleated cells in dermis | 12 (75) | 9 (33) | 18 (67) |
| Dermal inflammation (mild) | 8 (50) | 4 (15) | 10 (37) |
| Dermal inflammation (florid) | 1 (6) | 0 | 3 (11) |
| Pagetoid spread (mild) | 4 (25) | 5 (19) | 11 (41) |
| Pagetoid spread (florid) | 11 (69) | 20 (74) | 15 (56) |
| Density of junctional melanocytes (mild) | 7 (44) | 11 (41) | 15 (56) |
| Density of junctional melanocytes (florid) | 7 (44) | 12 (44) | 7 (26) |
RCM, reflectance confocal microscopy; DM, desmoplastic melanoma; HC, honeycomb.
Table 5.
Univariate exact logistic regression analyses for the RCM features comparing DM to the other melanoma controls, and the combined invasive melanoma group compared to the MIS group. All P values are two-sided. (DM n=16, melanoma in situ n=27, other invasive melanoma n=27). Significant values bolded (P <0.05)
| RCM Feature | DM vs entire control group | DM vs melanoma in situ | DM vs other invasive melanoma | Combined invasive melanoma vs MIS | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Regular HC pattern | 1.62 (0.44 – 6.81) | 0.608 | 1.10 (0.25 – 5.32) | 1 | 2.32 (0.55 – 11.02) | 0.319 | 0.64 (0.20 – 1.91) | 0.517 |
| Atypical HC | 1.33 (0.37 – 5.12) | 0.844 | 1.53 (0.37 – 6.73) | 0.720 | 1.14 (0.27 – 5.04) | 1 | 1.41 (0.48 – 4.18) | 0.644 |
| Broadened HC | 0.81 (0.21 – 2.90) | 0.948 | 1.02 (0.23 – 4.33) | 1 | 0.65 (0.15 – 2.69) | 0.720 | 1.34 (0.45 – 4.10) | 0.735 |
| Diamond-shaped HC | # | # | # | # | ||||
| Cobblestone pattern | 2.23 (0.31 – 13.32) | 0.523 | 1.32 (0.17 – 9.18) | 1 | 5.74 (0.42 – 326.77) | 0.275 | 0.59 (0.10 – 3.52) | 0.735 |
| Atypical cobblestone pattern | 0.38 (0 – 2.15) | 0.394 | 0.42 (0 – 2.86) | 0.474 | 0.42 (0 – 2.86) | 0.474 | 0.61 (0.08 – 4.88) | 0.850 |
| Atypical cobblestone pattern with small nucleated cells | 0.62 (0 – 3.80) | 0.690 | 0.683 (0 – 5.87) | 0.777 | 0.68 (0 – 5.87) | 0.777 | 0.61 (0.04 – 8.97) | 1 |
| Epidermal disarray | 1.42 (0.36 – 5.22) | 0.759 | 0.88 (0.20 – 3.66) | 1 | 2.58 (0.52 – 13.61) | 0.309 | 0.51 (0.16 – 1.59) | 0.287 |
| HC atypical and disarray | 2.57 (0.55 – 11.18) | 0.266 | 1.97 (0.37 – 10.67) | 0.554 | 3.52 (0.57 – 26.85) | 0.219 | 1.01 (0.25 – 4.43) | 1 |
| Pagetoid cells | ## | ## | ## | ## | ||||
| Widespread pagetoid infiltration | 1.78 (0.50 – 6.86) | 0.470 | 1.53 (0.37 – 6.73) | 0.720 | 2.05 (0.50 – 9.06) | 0.408 | 0.97 (0.33 – 2.84) | 1 |
| Round pagetoid cells | 0.31 (0.06 – 1.82) | 0.226 | 0.69 (0.12 – 4.16) | 0.892 | 0.09 (0 – 0.58) | 0.030 | 2.19 (0.42 – 12.26) | 0.447 |
| Dendritic pagetoid cells | 2.61 (0.47 - α) | 0.394 | 1.46 (0.17 – α) | 0.777 | 3.44 (0.55 – α) | 0.284 | 0.78 (0.07 – 5.94) | 1 |
| Pagetoid cells around follicular opening | 0.63 (0.06 – 3.53) | 0.892 | 0.51 (0.04 – 3.40) | 0.717 | 0.83 (0.07 – 6.66) | 1 | 0.57 (0.13 – 2.44) | 0.564 |
| Edged papillae | 1.22 (0.33 – 4.34) | 0.945 | 0.84 (0.20 – 3.43) | 1 | 1.82 (0.42 – 8.02) | 0.540 | 0.58 (0.19 – 1.73) | 0.394 |
| Non-edged papillae | 0.46 (0.13 – 1.69) | 0.289 | 0.43 (0.10 – 1.83) | 0.313 | 0.51 (0.12 – 2.13) | 0.448 | 0.65 (0.20 – 2.00) | 0.561 |
| Large interpapillary space | # | # | # | # | ||||
| Polycyclic papillae | 0.50 (0.05 – 2.72) | 0.643 | 0.42 (0.04 – 2.65) | 0.521 | 0.64 (0.05 – 4.57) | 0.950 | 0.56 (0.14 – 2.17) | 0.496 |
| Papillae enlarged and polycyclic | 0.54 (0.01 – 5.01) | 0.985 | 0.54 (0.01 – 7.47) | 1 | 0.54 (0.01 – 7.47) | 1 | 0.82 (0.13 – 6.11) | 1 |
| Non-visible papillae | 1.08 (0.25 – 4.08) | 1 | 1.29 (0.26 – 6.13) | 0.970 | 0.91 (0.19 – 4.05) | 1 | 1.37 (0.42 – 4.78) | 0.754 |
| Cell atypia | ## | ## | ## | ## | ||||
| Marked atypia | 0.93 (0.26 – 3.31) | 1 | 0.93 (0.23 – 3.81) | 1 | 0.93 (0.23 – 3.81) | 1 | 0.97 (0.33 – 2.84) | 1 |
| Sheet of cells | 1.16 (0.23 – 4.85) | 1 | 1.16 (0.20 – 6.12) | 1 | 1.16 (0.20 – 6.12) | 1 | 1.06 (0.30 – 4.11) | 1 |
| Junctional nest | 1.05 (0.21 – 4.32) | 1 | 0.95 (0.17 – 4.76) | 1 | 1.16 (0.20 – 6.12) | 1 | 0.87 (0.25 – 3.15) | 1 |
| Junctional clusters | 1.16 (0.23 – 4.85) | 1 | 1.16 (0.20 – 6.12) | 1 | 1.16 (0.20 – 6.12) | 1 | 1.06 (0.30 – 4.11) | 1 |
| Junctional thickening | 1.13 (0.02 – 15.32) | 1 | 1.71 (0.02 – 141.21) | 1 | 0.84 (0.01 – 17.39) | 1 | 1.93 (0.15 – 106.25) | 0.996 |
| Nests | 0.34 (0.03 – 1.78) | 0.292 | 0.51 (0.04 – 3.40) | 0.717 | 0.25 (0.02 – 1.47) | 0.162 | 1.35 (0.39 – 5.11) | 0.812 |
| Dense nest | 0.48 (0 - 2.76) | 0.523 | 0.42 (0 – 2.86) | 0.474 | 0.68 (0 – 5.87) | 0.777 | 0.40 (0.03 – 3.71) | 0.575 |
| Dishomogenous nest | 0.46 (0.05 – 2.42) | 0.537 | 0.83 (0.07 – 6.66) | 1.000 | 0.29 (0.03 – 1.76) | 0.247 | 1.96 (0.50 – 9.51) | 0.446 |
| Sparse nest | 0.30 (0.01 – 2.42) | 0.442 | 0.39 (0.01 – 4.47) | 0.751 | 0.24 (0.01 – 2.31) | 0.349 | 1.12 (0.25 – 5.80) | 1 |
| Cerebriform nest | 0.86 (0 – 5.87) | 0.906 | # | 0.42 (0 – 2.86) | 0.474 | 2.50 (0.37 – α) | 0.451 | |
| Plump bright cells | 4.05 (0.80 – 40.37) | 0.111 | 4.65 (0.80 – 50.33) | 0.102 | 3.41 (0.57 – 37.39) | 0.247 | 1.98 (0.63 – 6.32) | 0.287 |
| Plump bright cells in large aggregation within papillary dermis | 1.46 (0.28 – 6.30) | 0.802 | 1.45 (0.24 – 8.27) | 0.892 | 1.45 (0.24 – 8.27) | 0.892 | 1.16 (0.30 – 5.03) | 1 |
| Vessels: visible | 1.43 (0.33 – 5.56) | 0.781 | 1.97 (0.37 – 10.67) | 0.554 | 1.08 (0.22 – 4.93) | 1 | 1.89 (0.53 – 7.80) | 0.421 |
| Vertical vessel within the papillae | 3.45 (0.04 – 282.45) | 0.815 | 1.69 (0.09 – α) | 0.744 | 1.71 (0.02 – 141.21) | 1 | 1.54 (0.18 – α) | 0.748 |
| Horizontal vessel | 1.46 (0.28 – 6.30) | 0.802 | 1.45 (0.24 – 8.27) | 0.892 | 1.45 (0.24 – 8.27) | 1 | 1.16 (0.30 – 5.03) | 1 |
| Linear telangiectasia-like horizontal vessel | 0.66 (0.01 – 6.57) | 1 | 0.84 (0.01 – 17.39) | 1 | 0.54 (0.01 – 7.47) | 1 | 1.28 (0.17 – 15.11) | 1 |
| Convoluted glomerular-like vessel | 0.84 (0.02 – 9.33) | 1 | 1.71 (0.02 – 141.21) | 1 | 0.54 (0.01 – 7.47) | 1 | 2.63 (0.24 – 136.29) | 0.710 |
| Hyporeflective pagetoid cells | 0.54 (0.01 - 5.01) | 0.985 | 0.39 (0.01 – 4.47) | 0.751 | 0.84 (0.01 – 17.39) | 1 | 0.44 (0.06 – 2.83) | 0.506 |
| Spindle cells in superficial dermis | 3.82 (1.01 – 14.90) | 0.049 | 5.48 (1.11 – 32.36) | 0.034 | 2.78 (0.64 – 12.78) | 0.205 | 3.03 (0.81 – 14.32) | 0.113 |
| Nucleated cells in dermis | 2.96 (0.77 – 14.19) | 0.135 | 5.73 (1.27 – 31.80) | 0.019 | 1.49 (0.32 – 8.17) | 0.827 | 4.51 (1.47 – 14.80) | 0.006 |
| Dermal inflammation | 2.75 (0.77 – 10.36) | 0.135 | 1.94 (0.36 – 3.73) | 0.012 | 1.37 (0.34 – 5.79) | 0.844 | 5.87 (1.62 – 27.34) | 0.004 |
| Pagetoid spread | 0.88 (0.07 - 49.37) | 1 | 1.20 (0.06 – 75.45) | 1 | 0.59 (0.01 – 48.28) | 1 | 1.63 (0.11 – 23.78) | 1 |
| Junctional melanocytes | 1.39 (0.24 – 14.75) | 1 | 1.21 (0.15 – 15.05) | 1 | 1.58 (0.22 – 18.70) | 0.950 | 0.90 (0.17 – 4.01) | 1 |
RCM, reflectance confocal microscopy; DM, desmoplastic melanoma; OR, odds ratio; CI, confidence interval; HC, honeycomb.
indicates the RCM variable was absent in all cases in both groups, so no OR available.
indicates the RCM variable was present in all cases in both groups, so no OR available.
RCM-histology correlation
From the 14 DM cases that had histopathological slides available for central review, the percentage of desmoplasia in the dermal component was 100% for 6 cases, and less than 100% for 8 cases. Neurotropism was present in 4 cases. Using these 14 DM cases to test the RCM-histopathological correlation for the 5 analogous RCM-histological features described in Table 2, there was no agreement or poor agreement on Cohen ĸ statistics. The percentage of total concordant evaluations, (if variables were assessed only as present or absent), was 79% for density of junctional melanocytes, 71% for pigment incontinence, 50% for pagetoid spread, 43% for spindle cells within 0.3mm of the surface, and 36% for dermal inflammation. The RCM presence of spindle cells in the superficial dermis showed moderate correlation with invasive melanomas that featured spindle cells as a predominant cell type, if a predominant cell type was specified or available (n=34, ĸ=0.428 P=0.008).
Discussion
In the largest series describing dermoscopic features of DM (n=37) by Jaimes et al.5, all DM featured at least 1 melanoma-specific structure, with the most common one being atypical vascular structures (81%). A smaller study (n=6 DM) also identified one melanoma-specific structure in all DM cases.7 Our results, which also revealed a melanoma-specific structure on dermoscopy being found in all of our DM cases, validates this earlier research, and confirms that dermoscopy may be helpful in guiding the clinician towards a biopsy for lesions that ultimately result in DM diagnosis.
Both our study and the Jaimes et al.5 study found a high prevalence of atypical vascular structures in DM. This seemed to be one of the main differences when compared to general non-specified melanoma populations14,15, which had much lower frequencies of vascular structures. However, this difference could be related to Breslow thickness differences, as this dermoscopic feature became more frequent in the general melanoma population with increasing Breslow thickness (up to 47% for Breslow thickness >0.75mm14), and our study had a mean Breslow thickness of 3.3mm (standard deviation 2.36mm) in the DM population and the Jaimes et al.5 study had a mean Breslow thickness of 3.38mm in their DM population (range 0.5–12.0mm).
Previous RCM scoring systems developed in larger series for melanoma diagnosis8 and LM diagnosis13 demonstrated similar sensitivities at relevant cut-off scores (≥3 RCM score, ≥2 LM score) to those found in our study.
The additional examination of lesions with RCM following dermoscopy may possibly improve the ability to distinguish DM from MIS. While common DM dermoscopy features such as atypical vascular structures and milky red areas may suggest invasive melanoma over MIS14,16, our findings also suggest that the RCM features of dermal inflammation (Fig. 2) (OR 1.94), in addition to spindle cells in the superficial dermis (Fig. 3) (OR 5.48), and nucleated cells in the dermis (OR 5.73) may be useful to identify DM compared to non-DM MIS.
Figure 2.
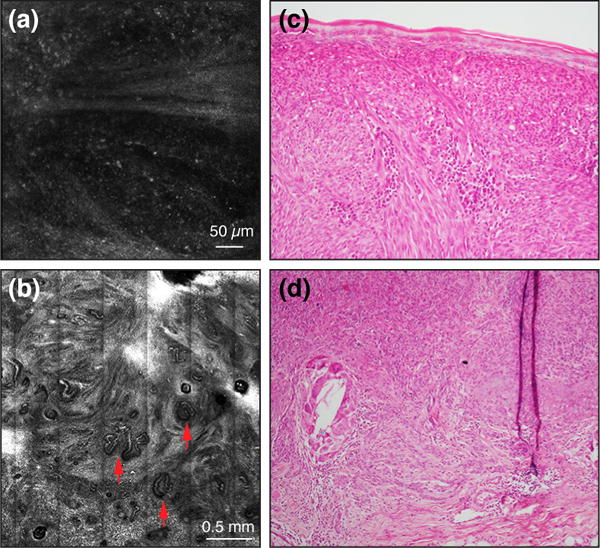
RCM images showing a diffuse infiltrate of small moderately refractile cells amongst collagen bundles in the superficial dermis (a), and convoluted vessels (red arrows) (b); the later is a known feature of thick melanoma (>1mm thickness).10 Histopathology images (c, d) of the same case revealed a patchy inflammatory infiltrate in a mixed nodular/desmoplastic type melanoma (only non-DM component shown), with a 2.15mm Breslow thickness (x100 original magnification).
Figure 3.
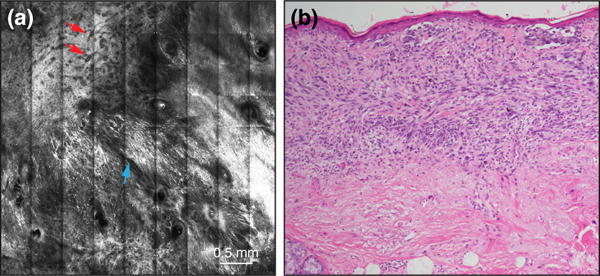
(a) A RCM mosaic image, showing a florid proliferation of spindle cells in the superficial dermis (blue arrow), and large branching vessels (red arrows). (b) Histopathology of the same case revealed a diffuse proliferation of spindle cells in the superficial dermis, correlating to RCM, in a mixed desmoplastic/lentigo maligna melanoma, with a 1.3mm Breslow thickness (x100 original magnification).
Univariate analysis indicated that the RCM features of nucleated cells in the dermis and dermal inflammation were both significant to distinguish any invasive type of melanoma from MIS in our series.
While our study showed that spindle cells in the superficial dermis on RCM may provide a clue towards DM diagnosis irrespective of whether the melanoma is in situ or invasive, this feature alone is not reliable, given it was only detected in 50% of DM cases, may be present with other melanoma invasive subtypes, and also in other types of neoplasms (e.g. Spitz nevi). Interestingly, spindle cells in the superficial dermis on RCM showed a moderate correlation to spindle cells as the predominant cell type on histopathology within the group of invasive melanomas in our series, but when examined amongst DMs, demonstrated poor correlation to histopathology. This may imply that when spindle cells are more abundant (or less sparsely distributed), the RCM-histopathology correlation improves. In potential support of this, a study on Spitz nevi showed good correlation between spindled cells on RCM and histopathology (n=40, ĸ=0.400).9
In general, there were poor correlations between analogous RCM and histopathological characteristics evaluated amongst the 14 DM cases. This could be attributed to a number of things. Firstly, 3 of these 5 features analysed were from the dermis, at the limit of RCM imaging capabilities. Other studies have shown good correlation between RCM and histopathology for melanocytic pathologies under different circumstances,9,17 but difficulties with correlation in the dermal layer.18 Secondly, there was no guarantee that RCM images were taken from where the DM was identified histologically within the specimen. While this would be desirable in a prospective study design, because DM is rare, it may be difficult to undertake. Th Thirdly, it may reflect the small number of DM patients, which could skew the statistics. In addition, a single RCM rater was a limitation of our study.
The other limitations of our study, included the retrospective design, which did not allow for a standardized protocol for acquisition of RCM images, and that RCM technology has improved over the ten-year accrual period to find these DM cases.
In conclusion, a melanoma-specific structure on dermoscopy, particularly atypical vascular structures, along with recognized RCM features for melanoma (such as pagetoid cells, cell atypia, and nucleated cells in the dermis)8,10 are useful for the clinical identification of DM. When the RCM features of dermal inflammation and nucleated cells in the dermis are present, this favours invasive melanoma over MIS. Whereas, the presence of abundant spindled cells intermingled with collagen fibers can be considered a specific clue for DM diagnosis, this feature is not always observable, probably because of the limited depth of exploration of RCM. Further studies are required to validate these findings. Should this be the case, these features could be used to aid RCM targeted biopsies and RCM follow-up of MIS treated non-invasively.
Acknowledgments
Funding Support: Dr Nigel G. Maher received the Australian Postgraduate Award from the University of Sydney for his Master of Philosophy degree.
The research at the Melanoma Unit in Barcelona is partially funded by Spanish Fondo de Investigaciones Sanitarias (PI15/00716 and grants PI15/00956); Biomedical Network Research on Rare Diseases (CIBERER) of the Instituto de Salud Carlos III, Spain; AGAUR 2014 SGR 603 of the Catalan Government, Spain; European Commission under the 6th Framework Programme, Contract No.LSHC-CT-2006-018702 (GenoMEL) and by the European Commission under the 7th Framework Programme, Diagnoptics; The National Cancer Institute (NCI) of the US National Institute of Health (NIH) (CA83115) and a grant from “Fundació La Marató de TV3, 201331-30”, Catalonia, Spain.
Footnotes
Conflict of Interest Disclosures: None reported.
References
- 1.Quinn M, Crotty K, Thompson J, Coates A, O’Brien C, McCarthy W. Desmoplastic and desmoplastic neurotropic melanoma: experience with 280 patients. Cancer. 1998;83(6):1128–1135. [PubMed] [Google Scholar]
- 2.Busam K, Mujumdar U, Hummer A, et al. Cutaneous desmoplastic melanoma: reappraisal of morphologic heterogeneity and prognostic factors. Am J Surg Pathol. 2004;28(11):1518–1525. doi: 10.1097/01.pas.0000141391.91677.a4. [DOI] [PubMed] [Google Scholar]
- 3.Murali R, Shaw H, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010;116(17):4130–4138. doi: 10.1002/cncr.25148. [DOI] [PubMed] [Google Scholar]
- 4.George E, McClain S, Slingluff C, Poslissar N, Patterson J. Subclassification of desmoplastic melanoma: pure and mixed variants have significantly different capacities for lymph node metastasis. J Cutan Pathol. 2009;36(4):425–432. doi: 10.1111/j.1600-0560.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- 5.Jaimes N, Chen L, Dusza S, et al. Clinical and Dermoscopic Characteristics of Desmoplastic Melanomas. JAMA Dermatol. 2013;149(4):413–421. doi: 10.1001/jamadermatol.2013.2248. [DOI] [PubMed] [Google Scholar]
- 6.Lens M, Newton-Bishop J, Boon A. Desmoplastic malignant melanoma: a systematic review. Br J Dermatol. 2005;152(4):673–678. doi: 10.1111/j.1365-2133.2005.06462.x. [DOI] [PubMed] [Google Scholar]
- 7.Debarbieux S, Ronger-Salve S, Dalle S, Balme B, Thomas L. Dermoscopy of desmoplastic melanoma: report of six cases. Br J Dermatol. 2008;159(2):360–363. doi: 10.1111/j.1365-2133.2008.08687.x. [DOI] [PubMed] [Google Scholar]
- 8.Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127(12):2759–2765. doi: 10.1038/sj.jid.5700993. [DOI] [PubMed] [Google Scholar]
- 9.Pellacani G, Longo C, Ferrara G, et al. Spitz nevi: In vivo confocal microscopic features, dermatoscopic aspects, histopathologic correlates, and diagnostic significance. J Am Acad Dermatol. 2009;60(2):236–247. doi: 10.1016/j.jaad.2008.07.061. [DOI] [PubMed] [Google Scholar]
- 10.Guitera P, Menzies S, Longo C, Cesinaro A, Scolyer R, Pellacani G. In Vivo Confocal Microscopy for Diagnosis of Melanoma and Basal Cell Carcinoma Using a Two-Step Method: Analysis of 710 Consecutive Clinically Equivocal Cases. J Invest Dermatol. 2012;132(10):2386–2394. doi: 10.1038/jid.2012.172. [DOI] [PubMed] [Google Scholar]
- 11.Losi A, Longo C, Cesinaro A, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171(1):48–54. doi: 10.1111/bjd.12781. [DOI] [PubMed] [Google Scholar]
- 12.Ardigò M, Agozzino M. The Semiology and Patterns of Inflammatory Skin Conditions. In: Hofmann-Wellenhof R, Pellacani G, Malvehy J, Soyer P, editors. Reflectance Confocal Microscopy for Skin Diseases. Vol. 2012. Springer; Berlin Heidelberg: pp. 351–352. [Google Scholar]
- 13.Guitera P, Pellacani G, Crotty K, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130(8):2080–2091. doi: 10.1038/jid.2010.84. [DOI] [PubMed] [Google Scholar]
- 14.Pizzichetta M, Argenziano G, Talamini R, et al. Dermoscopic criteria for melanoma in situ are similar to those for early invasive melanoma. Cancer. 2001;91(5):992–997. [PubMed] [Google Scholar]
- 15.Argenziano G, Soyer P, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48(5):679–693. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 16.Silva V, Ikino J, Sens M, Nunes D, Di Giunta G. Dermoscopic features of thin melanomas: a comparative study of melanoma in situ and invasive melanomas smaller than or equal to 1mm. An Bras Dermatol. 2013;88(5):712–717. doi: 10.1590/abd1806-4841.20132017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellacani G, Longo C, Malvehy J, et al. In vivo confocal microscopic and histopathologic correlations of dermoscopic features in 202 melanocytic lesions. Arch Dermatol. 2008;144(12):1597–1608. doi: 10.1001/archderm.144.12.1597. [DOI] [PubMed] [Google Scholar]
- 18.Farnetani F, Scope A, Braun R, et al. Skin Cancer Diagnosis With Reflectance Confocal Microscopy: Reproducibility of Feature Recognition and Accuracy of Diagnosis. Jama Dermatol. 2015;151(10):1075–1080. doi: 10.1001/jamadermatol.2015.0810. [DOI] [PubMed] [Google Scholar]


