Abstract
Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells contribute to the body’s immune defenses. Current chimeric antigen receptor (CAR)-modified T cell immunotherapy shows strong promise for treating various cancers and infectious diseases. Although CAR-modified NK cell immunotherapy is rapidly gaining attention, its clinical applications are mainly focused on preclinical investigations using the NK92 cell line. Despite recent advances in CAR-modified T cell immunotherapy, cost and severe toxicity have hindered its widespread use. To alleviate these disadvantages of CAR-modified T cell immunotherapy, additional cytotoxic cell-mediated immunotherapies are urgently needed. The unique biology of NK cells allows them to serve as a safe, effective, alternative immunotherapeutic strategy to CAR-modified T cells in the clinic. While the fundamental mechanisms underlying the cytotoxicity and side effects of CAR-modified T and NK cell immunotherapies remain poorly understood, the formation of the immunological synapse (IS) between CAR-modified T or NK cells and their susceptible target cells is known to be essential. The role of the IS in CAR T and NK cell immunotherapies will allow scientists to harness the power of CAR-modified T and NK cells to treat cancer and infectious diseases. In this review, we highlight the potential applications of CAR-modified NK cells to treat cancer and human immunodeficiency virus (HIV), and discuss the challenges and possible future directions of CAR-modified NK cell immunotherapy, as well as the importance of understanding the molecular mechanisms of CAR-modified T cell- or NK cell-mediated cytotoxicity and side effects, with a focus on the CAR-modified NK cell IS.
Keywords: natural killer cell, chimeric antigen receptor, immunotherapy, immunological synapse, HIV, cancer
INTRODUCTION
Natural killer (NK) cells were discovered in the 1970s (Herberman et al., 1975a, b; Kiessling et al., 1975a, b) but are not considered a main research area in the field of immunology (Yokoyama, 2008). For decades, NK cells have lived in the shadow of T cells and other immune cells. In the early days of NK cell discovery, many immunologists did not appreciate the importance of NK cells to the body’s defense system. However, the essential roles of NK cells in the immune system are revealed in patients with NK deficiency (Orange, 2013), who have increased rates of malignancy (Orange, 2013; Morvan and Lanier, 2016) and are susceptible to herpesvirus infections, including varicella pneumonia, disseminated cytomegalovirus, and herpes simplex virus (Biron et al., 1989). Similar results were described in mice with impaired NK activity (Talmadge et al., 1980; Morvan and Lanier, 2016), which highlights the importance of NK cells in the immune system.
Chimeric antigen receptor (CAR)-modified T cell therapy has become a promising immunotherapeutic strategy for the treatment of blood cancers (Porter et al., 2011; Kim et al., 2016; Maude and Barrett, 2016) and has gained the significant attention of researchers in both academia and industry (Glienke et al., 2015). In 1989, Gross and colleagues described the concept of expressing antibody in a cytotoxic T cell hybridoma (Gross et al., 1989). Progressive advances in construction of chimeric T cell receptors, including introduction of a single costimulatory molecule, in the past 25-year development by several groups on the basis of this concept have led to rapid progress on CAR Therapy. For example, anti-CD19 CAR T cell therapy was granted “breakthrough therapy” by the United States Food and Drug Administration (FDA) in 2014 (Gill and June, 2015; Gill et al., 2016).
NK cells were originally described for their capacity to spontaneously kill tumor cells (Rosenberg et al., 1974; Herberman et al., 1975a, b; Kiessling et al., 1975a, b), which differ from T cells that require prior sensitization. As their name implies, NK cells kill susceptible target cells, such as virus-infected cells or tumor cells, without prior sensitization. Unlike T cells, the activation of NK cells is controlled by the integration of both stimulatory and inhibitory receptors (Bakker et al., 2000; Long et al., 2013). Similar to CAR-T technology, CAR-NK cell strategy involves isolating a patient’s own NK cells or expanding commercially available NK cell lines (e.g., NK92 cells), engineering these cells in a good manufacturing practice (GMP) laboratory to express CAR, which recognize a tumor-specific protein, and re-infusing the engineered NK cells back into the patient.
The cell biology and immunology of NK cells have been extensively discussed in other reviews (Lanier, 2005; Vivier et al., 2011; Lam and Lanier, 2016). This review focuses on CAR-modified NK cell immunotherapy and the CAR-modified NK immunological synapse (IS). This review is divided into the following five main sections: (1) the rationale for the development of CAR-modified NK cell-based immunotherapy; (2) CAR-modified NK cell-based immunotherapy to treat cancer; (3) CAR-modified NK cell-based immunotherapy to treat infectious diseases; (4) the CAR-modified NK cell IS; and (5) perspectives. The main messages that this review conveys are the following:
CAR-modified NK cells are a promising intervention for the treatment of cancer and infectious diseases.
The CAR-modified NK cell IS is important to the understanding of CAR-modified NK cell-mediated cytotoxicity and side effects.
THE RATIONALE FOR THE DEVELOPMENT OF CAR-MODIFIED NK CELL-BASED IMMUNOTHERAPY
In addition to T cell-mediated immunotherapy, the unique biology of NK cells makes them a valid tool for immunotherapy (Morvan and Lanier, 2016). Clinically, NK cells from peripheral blood can be defined as CD3-negative and CD56-positive peripheral blood mononuclear cells (PBMCs). There are several advantages of using NK cells as an immunotherapeutic strategy to treat cancer and infectious diseases, as described below.
NK cell activation does not require prior sensitization or human leukocyte antigen matching
It is well known that NK cell activation is controlled by the integration of stimulatory and inhibitory signals (Long et al., 2013). CTL activation requires a sensitization phase, in which unprimed T cells interact with antigen-presenting cells (APCs) to become activated lymphocytes. These activated CTLs then enter the effector phase in which they eliminate virus-infected target cells or tumor cells. However, NK cells can kill virus-infected target cells and tumor cells without prior sensitization. Specifically, if the signaling of an activating receptor, such as CD16 or natural killer group 2, member D (NKG2D), dominates, NK cells will kill target cells without prior sensitization, which provide the rapid, first-line defense mechanism. This feature of NK cell biology makes these cells an effective and valid tool for rapidly eliminating virus-infected cells or tumor cells, serving as the body’s first line of defense. Another feature of CTL-mediated killing is “major histocompatibility complex (MHC) restriction”, in which the T cell receptor (TCR) must recognize its self-MHC molecule that is expressed on antigen-specific target cells, such as virus-infected cells (Ada, 1994). However, NK cell-mediated cytotoxicity is “MHC unrestricted”, which means that NK cells kill target cells that don’t express MHC class I molecules, a guiding principle in the NK field, also known as the “missing self” hypothesis (Karre et al., 1986; Ljunggren and Karre, 1990). NK cell activation is controlled by the strength of the stimulatory receptor signal. The principle of CAR immune cell design matches the mechanism of NK cell activation. CAR-modified NK cells can provide strong activating signals by linking the antigen-specific single-chain variable fragment (scFv) domain with CD3zeta, an essential intracellular signaling molecule for NK cell activation (Lanier, 2005; Watzl and Long, 2010). Given the unique NK cell mechanism for killing target cells, CAR-modified NK cells provide attractive effector cells for immunotherapy.
NK cell killing does not require antigen-specific receptors, such as TCRs for CTLs
As described above, NK cell cytotoxicity is triggered by various germline-encoded stimulatory receptors (Vivier et al., 2008) and does not require the highly polymorphic TCR. A potential concern of genetically modified T cells with tumor- or virus-specific TCRs is “TCR mispairing” (Kershaw et al., 2005), which occurs when introduced alpha and beta TCR chains mispair with endogenous TCR chains. This process not only creates mismatched heterodimers of unknown specificity, but also reduces the cell-surface density of tumor- or virus-reactive TCRs (Aggen et al., 2012). To avoid “TCR mispairing”, Aggen and colleagues developed single-chain T cell receptor variable fragment (scTv), which links the variable domains of the alpha and beta TCR chains by a flexible linker, to generate high-affinity, stable TCR for MHC complexes associated with cancer and HIV (Aggen et al., 2012). Therefore, it is possible to modify NK cells with an antigen-specific scTv gene without concerns of “TCR mispairing”, a phenomenon commonly found in TCR-modified CTLs (Kershaw et al., 2005). Therefore, genetically modified NK cells expressing scTv are easier and more feasible to generate than T cells expressing exogenous TCRs.
NK cell immunotherapy has less severe side effects
In contrast to T cells, CAR-modified NK cells show less severe side effects, such as graft-versus-host disease (GvHD), because donor NK cells usually do not attack non-hematopoietic tissues such as liver, kidney, muscle, and lung. A number of clinical trials showed that NK cell infusion has less severe GvHD than does T cell infusion. This clinical observation could also be related to the unique cell biology and immunology features of NK cells. For example, compared to T cells, conventional NK cells have a shorter life span, which can mitigate the risk of GvHD development in leukemia patients treated with NK cells (Miller et al., 2005; Curti et al., 2011). In addition to a shorter life span, NK cell expansion is tightly controlled by constitutively expressed inhibitory receptors, such as killer immunoglobulin-like receptor (KIR), CD94/ natural killer group 2A (NKG2A), and other inhibitory receptors (Long, 2008). These features of NK cells may explain why NK cells have less severe GvHD during NK cell infusion, compared with T cell infusion. However, a direct comparison between CAR-modified NK cells and CAR-modified T cells has not been performed in vivo.
CAR-modified NK cells can be potentially used as an “off-the-shelf” universal CAR product
A pressing issue in the field of immunotherapy is whether an “off-the-shelf” universal CAR product can be developed. CAR-modified T cell products from individuals are costly and time consuming to prepare. Generation of “off-the-shelf” CAR-modified T cell products will significantly reduce the cost of immunotherapy. Using the clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein 9 technique (Cong et al., 2013; Cong and Zhang, 2015), as well as other gene-editing technologies, to knockout endogenous TCRs and human leukocyte antigen (HLA) class I molecules for universal CAR-modified T cell generation is still in the preclinical phase of investigation (Ren et al., 2016; Liu et al., 2017b). Most of these strategies are in the concept phase. However, CAR-modified NK cell lines such as NK92 may lead to the development of feasible, “off-the-shelf” CAR products in the near future.
Sufficient numbers of NK cells can be harvested from peripheral blood
A critical aspect of successful immunotherapy is to rapidly generate sufficient numbers of CAR-modified cells in vitro, which requires at least one million cells for expansion ex vivo. In general, there are sufficient numbers of NK cells that can be directly isolated from peripheral human blood. Around 10%–15% of PBMCs in the buffy coat layer are CD3-negative and CD56-positive NK cells. We usually can isolate between 10–20 million NK cells per buffy coat layer in each healthy individual. Immobilized apheresis products contain 5%–15% NK cells. To isolate NK cells, CD3+ cell depletion of PBMCs is commonly performed, followed by CD56+ cell enrichment using immunomagnetic bead separation with medical devices and clinical-grade reagents; these methods are feasibly conducted in the clinic due to the sufficient numbers of NK cells that can be isolated directly from peripheral blood.
Functional NK cell lines are available
Compared to CAR-modified T cell-mediated immunotherapy, an advantage of NK cell-based immunotherapy is that functional, immortal NK cell lines are available. There are a number of functional NK cell lines that are cytotoxic and produce cytokines, such as NK92 (Gong et al., 1994), NKL (Robertson et al., 1996), KHYG-1 (Yagita et al., 2000), and YTS (Cohen et al., 1999; Klingemann et al., 2016). Among these NK cell lines, NK92 is the most promising cell line for clinical applications. NK92-mediated immunotherapy is now undergoing phase I/II clinical trials (Arai et al., 2008; Tonn et al., 2013). Commonly, NK92 cells must be irradiated prior to infusion to prevent permanent engraftment. The amount of irradiation required is around 10 Gy. The dose of irradiated NK92 infusion can be up to 1010 NK92 cells/m2. Importantly, irradiated NK92 cells have been proven safe for infusion in patients, as demonstrated by several NK92 clinical trials (NCT00900809, NCT00990717, NCT00995137, and NCT01974479). In contrast to CAR-modified T-mediated immunotherapy, there are few functional CTL cell lines available that can be used in clinical trials. Antigen-specific CTL clones can be expanded ex vivo for 2–3 months. After CTL clone expansion in vitro, many features of the CTL clones are altered, limiting their clinical application. In addition, there are few reports of the clinical applications of CTL clones to treat cancer and infectious diseases. Although several advantages of NK cells described above regarding NK-based immunotherapy, abnormal NK cell number and cytolytic functions have been proposed in various cancers (Costello et al., 2002; Farnault et al., 2012). Using functional CAR-modified NK cell lines is an important, alternative strategy to treat cancer and infectious diseases. In conclusion, to alleviate some disadvantages of CAR-modified T cell immunotherapy, additional NK-based CAR immunotherapy can be an alternative and effective approach for some patients. However, in vivo direct comparison between CAR-T and CAR-NK on the cytotoxicity, manufacture speed, proliferation capability, persistence, side effects etc., is urgently needed.
CAR-MODIFIED NK CELL-BASED IMMUNOTHERAPY TO TREAT CANCER
Cancer is the leading cause of death worldwide: An estimated 8.2 million people die each year from cancer. A major public health problem in the United States, cancer is the second-leading cause of death (Siegel et al., 2016). In 2016, 1,685,210 new cancer cases and 595,690 cancer deaths were projected to occur in the United States (DeSantis et al., 2016; Siegel et al., 2016; Torre et al., 2016).
The clinical investigation of CAR-modified NK cell-based immunotherapy has been intensively conducted for several types of cancer (Rezvani and Rouce, 2015). Similar to CAR-T cell based immunotherapy, genetically modified NK cells using various CAR molecules to redirect different antigen specificity has been discussed by other reviews (Glienke et al., 2015; Hermanson and Kaufman, 2015; Rezvani and Rouce, 2015). This section will focus on the use of the CAR-modified NK92 cell line. Currently, CAR-modified NK92 cell line is used as effector cells for various cancer treatments, as detailed below:
CAR-modified NK cells to treat acute lymphoblastic leukemia
CD5 is highly expressed in T cell acute lymphoblastic leukemia (T-ALL) and peripheral T cell lymphoma. A recent study showed that CD5-CAR-modified NK92 cells can kill a variety of T cell leukemia and lymphoma cell lines as well as primary tumor cells in vitro and in xenograft mouse models of T-ALL (Chen et al., 2017).
In addition to T-ALL, CD19-CAR-modified NK cell-based immunotherapy can be used to treat primary chronic lymphocytic leukemia (CLL) (Boissel et al., 2013), acute myeloid leukemia (AML, ClinicalTrials.gov.NCT00995137), myelodysplastic syndromes (Gleason et al., 2014), and B cell leukemia and lymphoma (Oelsner et al., 2017). The cytotoxicity of NK92 cells expressing CD20-CAR against primary CLL cells is superior to the cytotoxicity of NK92 cells expressing IgG Fcγ receptor III (FcγRIII, also known as CD16) combined with anti-CD20 monoclonal antibodies, such as rituximab or ofatumumab (Boissel et al., 2013).
Interestingly, trogocytosis can be used as a non-viral method to modify NK cells. Conventionally, immune cells can be directly modified using CAR viral particles. However, the authors used anti-CD19-CAR particles to transfect the K562 cell line (the first human immortalized myelogenous leukemia line with MHC class I deficiency). After mixing CD19-CAR-modified K562 cells with human primary NK cells isolated from PBMCs, CD19-CAR protein was transferred from CD19-CAR-modified K562 cells into NK cells via trogocytosis. The transferred CD19-CAR-modified NK cells functionally kill B cell acute lymphoblastic leukemia (B-ALL) cell lines and primary B-ALL cells derived from patients (Cho et al., 2014). This novel strategy could be a potential valuable therapeutic approach for modifying NK cells.
CAR-modified NK cells to treat glioblastoma and neuroblastoma
It is well known that CAR-modified T cells face a unique set of challenges during the targeting of solid tumors (Gilham et al., 2012). The development of CAR-modified NK cells must overcome similar obstacles. Glioblastoma is one of the most lethal primary brain malignancies in adults and children, because of its highly invasive and metastatic characteristics (Magana-Maldonado et al., 2016). Neuroblastoma is a neuroendocrine tumor of early childhood and is the most common extracranial solid tumor that occurs in children (Matthay et al., 2016). It has been reported that NK92 cells have been developed to treat both glioblastoma and neuroblastoma in vitro. These cells have been modified to target neuroblastoma using a GD2 (disialoganglioside)-specific CAR (Esser et al., 2012) and to target glioblastoma using either an ErbB2 (origin in the ERB-B gene responsible for avian erythroblastosis virus)-CAR (Zhang et al., 2016) or an EGFR-CAR (Han et al., 2015). Therefore, it will be of interest to determine whether CAR-modified NK92 cells can treat both glioblastoma and neuroblastoma in clinical trials.
CAR-modified NK cells to treat breast cancer
Breast cancer is the most common cancer of females in the U.S. (DeSantis et al., 2014). An adoptive cancer immunotherapy using CAR-modified NK92 cells has been rapidly developed. A stable NK92 cell line expressing an anti-human epidermal growth factor receptor 2 (HER2, also known as ErbB2)-CAR exhibited specific antitumor activity in vivo using an experimental non-obese diabetic (NOD) severe combined immunodeficiency (SCID) gamma (NSG) lung metastasis model (Schonfeld et al., 2015). Similarly, the combination of an EGFR-CAR-modified NK92 cell line therapy with the oncolytic herpes simplex virus 1 (oHSV-1) is a promising strategy for the treatment of EGFR-positive breast cancer that has metastasized to the brain (Chen et al., 2016).
CAR-modified NK cells to treat multiple myeloma
Multiple myeloma (MM) is an incurable hematological malignancy that results from genetic mutations that occur during the process whereby B lymphocytes differentiate into plasma cells (Kyle and Rajkumar, 2008). Currently, a number of CAR-modified T cells have been developed for the treatment of MM (Liu et al., 2017a; Luetkens et al., 2017). However, few studies have reported using CAR-modified, primary NK cells for the treatment of MM. Current strategies using CAR-modified NK cell products to treat MM are focused on the CAR-modified NK92 cell line.
The cell surface glycoprotein CD2 subset 1 (CS1, also known as CRACC, SLAMF7, CD319, or 19A24) is a surface protein that is highly expressed on MM cells (Hsi et al., 2008; Tai et al., 2008). A previous study showed that CS1-CAR-modified NK92 cells inhibited MM tumor growth and prolonged survival of tumor-bearing NSG mice (Chu et al., 2014). CD138, also known as syndecan-1, is a primary diagnostic marker for MM (Akl et al., 2015). Therefore, CD138-CAR-modified NK92 cells have been used to treat MM in non-obese diabetic mice with severe combined immunodeficiency (Jiang et al., 2014).
CAR-modified NK cells to treat prostate cancer metastases
Instead of using a conventional CAR containing the CD3zeta domain, a new CAR containing DNAX activation protein 12 (DAP12) has been proposed to modify NK cells (Topfer et al., 2015). Compared to CD3zeta, DAP12 is a signaling adaptor molecule involved in the signal transduction of stimulatory NK cell receptors, such as NKG2C (Lanier et al., 1998a), NKP44 (Campbell et al., 2004), KIR3DS1 (Carr et al., 2007), and KIR2DS1/2/5 (Lanier et al., 1998a, b; Smith et al., 1998; Della Chiesa et al., 2008; Hayley et al., 2011). Therefore, a DAP12-based, anti-prostate stem cell antigen CAR could be used to modify NK cells for the treatment of prostate cancer (Topfer et al., 2015). The data demonstrate that the use of NK cells modified with DAP12-based CARs is a promising approach for adoptive immunotherapy.
In conclusion, in addition to enhancing antibody-dependent cell-mediated cytotoxicity (ADCC) and blocking inhibitory receptor functions using KIR and other inhibitory receptors antibodies, modifying NK92 cell line to target various tumors has been a major, current effort in the field of NK cell-based immunotherapy.
CAR-MODIFIED NK CELL-BASED IMMUNOTHERAPY TO TREAT INFECTIOUS DISEASES
Although great advances in human immunodeficiency virus (HIV, one of the major human pathogens) treatment have been made in most developed countries, the HIV/acquired immune deficiency syndrome (AIDS) pandemic continues to be a major public health problem in the majority of developing countries. In this section, we focus on the potential applications of CAR-modified NK cells for the treatment of HIV infections.
Eradication of HIV from infected individuals remains a major medical challenge (Siliciano, 2014; Churchill et al., 2016; Mzingwane and Tiemessen, 2017). Although combination antiretroviral therapy (cART) has dramatically reduced HIV-related morbidity and mortality (Palella et al., 2006), it fails to eliminate HIV in vivo due to the persistence of replication-competent proviruses in long-lived, latently infected cells, also known as HIV reservoirs. Recently, the use of CAR-modified CTLs to target cancer has become a promising approach for cancer immunotherapy and represents a broad-based approach by which T cells can be engineered to overcome antigen restriction or mutation (Wang and Wang, 2017). To test whether CAR-modified CTLs can be redirected toward HIV-infected target cells, several groups have developed different CAR-modified T cells for this purpose (Liu et al., 2015; Zhen et al., 2015; Ali et al., 2016; Liu et al., 2016), including one of earliest CAR-modified T cell clinical trials designed to treat HIV (Yang et al., 1997). Although the field is extensively investigating CAR-modified T cells to treat chronic HIV infections, the use of CAR-modified NK cell-based immunotherapy to treat chronic HIV infections has several advantages, as described below.
NK cells can directly recognize HIV-infected target cells
The adaptive immune system, including T cells and B cells, plays an essential role in HIV control during the acute and chronic phases of infection (McMichael et al., 2010; Jones and Walker, 2016). However, an increasing body of evidence shows that NK cell dysfunction is closely associated with HIV disease progression (Alter and Altfeld, 2006; De Maria and Moretta, 2008; Iannello et al., 2008a, b; Alter and Altfeld, 2009; Fadda and Alter, 2011; Jost and Altfeld, 2012; Kramski et al., 2013; Hens et al., 2016; Scully and Alter, 2016).
HIV-infected target cells can upregulate the ligands recognized by NK cells (Richard et al., 2010). For example, HIV-infected primary NK cells upregulate unique long (UL) 16 (a human cytomegalovirus glycoprotein) binding protein (ULBP)-1 and -2, but not ULBP-3, MHC class I polypeptide-related sequence (MIC)-A, or MIC-B (Ward et al., 2009). ULBP-1 and -2 can strongly induce NKG2D -mediated NK cell immune responses in humans (Ogasawara and Lanier, 2005; Bryceson and Ljunggren, 2008; Le Bert and Gasser, 2014).
NK cells can rapidly secret Interferon (IFN)-gamma to initiate a cellular anti-HIV response
The production of IFN-gamma by immune cells is an essential defense mechanism against many viral, bacterial, and parasitic infections (Borges da Silva et al., 2015). NK cells are a major innate source of IFN-gamma (Travar et al., 2016; Waggoner et al., 2016). Although CD4+ T cells are a major adaptive source of IFN-gamma, their ability to respond to infection is slower than that of NK cells. NK cells produce IFN-gamma rapidly during infections, which is essential to drive the differentiation of CD4+ T cells and induce adaptive immune responses. During HIV infections, NKG2A+ NK cells can quickly produce IFN-gamma to control HIV replication (Lisovsky et al., 2015), suggesting the important role of NK cells in the control of early HIV infection.
NK cells can kill HIV-infected target cells via ADCC
ADCC is one of the most important functions of NK cells. ADCC plays an important role in controlling HIV infections (Chung et al., 2008). For example, ADCC activity was modestly protective in the RV144 HIV vaccine (an estimated efficacy of 31.2% at preventing HIV infection among South African adults) trial (Haynes et al., 2012). Enhancing ADCC activity could be a potential strategy for HIV vaccine development.
NK cells can kill T follicular helper (Tfh) cells, a critical HIV reservoir
CD4+ PD-1high Tfh cells are a newly identified virus reservoir in HIV-1 patients. The size of HIV-1 reservoirs positively correlates with the numbers of PD-1-expressing cells (Chomont et al., 2009; Hatano et al., 2013), as PD-1 expression marks cells that are more likely to harbor HIV-1 (Chomont et al., 2009; Deeks et al., 2012). Tfh cells in lymph nodes (LNs) are PD-1 positive and serve as the major CD4+ T cell compartment for HIV-1 infection, replication, and production (Perreau et al., 2013). Thus, Tfh cells in LNs and peripheral blood (PB) are also likely to be a key cellular reservoir for latent HIV-1 (Vinuesa, 2012; Pallikkuth et al., 2015). Although PD-1 expression is a hallmark of exhausted T cells during chronic infections (Wherry, 2011), PD-1high Tfh cells do not experience exhaustion during chronic infections (Choi et al., 2013), indicating that they may have superior abilities to self-renew, resist apoptosis, and survive for extremely long periods of time during HIV-1 infection. Importantly, a recent study showed that NK cells can suppress CD4+ T cells and Tfh cells in a perforin-dependent manner during the first few days of infection (Rydyznski et al., 2015), resulting in a weaker germinal center (GC) response and diminished immune memory. One of current efforts in the search for an HIV cure includes disrupting GC formation, thereby reducing HIV reservoirs.
NK cell KIR is associated with HIV selection pressure
Effective NK cell responses impact HIV-1 progression (Fauci et al., 2005; Alter and Altfeld, 2006; Alter and Altfeld, 2009; Alter and Altfeld, 2011; Alter et al., 2011; Altfeld et al., 2011; Funke et al., 2011; Jost and Altfeld, 2012, 2013; Altfeld and Gale, 2015). Killer cell immunoglobulin like receptor, three Ig domains and short cytoplasmic tail 1 (KIR3DS1), in particular, appears to inhibit HIV-1 replication in vitro (Martin et al., 2002; Alter et al., 2007; Jost and Altfeld, 2013) and be associated with slower AIDS progression in HIV-1-infected patients (Martin et al., 2002; Pascal et al., 2007; Long et al., 2008). Interestingly, KIR3DS1+ NK cells express high levels of IFN-γ (indicating enhanced cytokine secretion) and CD107a (indicating enhanced degranulation and cytotoxicity) in adults who were recently infected with HIV-1 (Long et al., 2008). In summary, the combination of KIR on NK cells and host human leukocyte antigen (HLA) can affect HIV progression.
NK cells cannot be effectively infected by HIV
HIV primarily infects CD4+ T cells. Whether NK cells can be infected by HIV is controversial (Funke et al., 2011), although the consensus in the field is that HIV cannot effectively infect the majority of NK cells, because NK cells present in peripheral blood lack CD4 expression, and no proviral DNA can be detected in NK cells from HIV patients (Mavilio et al., 2003; Altfeld et al., 2011). A subpopulation of NK cells (<7%) was found to be sensitive to infection by HIV-1 in vitro (Chehimi et al., 1991; Scott-Algara et al., 1993; Valentin et al., 2002; Bernstein et al., 2009), but these data must be verified in vivo. In general, the majority of NK cells cannot be infected by HIV naturally, which makes NK cells attractive effector cells for the treatment of HIV. Importantly, NK cell lines that are currently used in clinical trials are CD4 negative, which means that these NK cell lines cannot be infected by HIV.
CAR-modified NK cell immunotherapy for HIV
Despite the increasing body of evidence showing NK cell involvement in the control of HIV infection, HIV can affect NK cell phenotype and function during HIV infection, including the cytokine/chemokine production, activation, and cytotoxicity of NK cell subsets. Therefore, genetically modified NK cells, designed to enhance innate immunity, are essential for the development of a novel strategy to control infectious diseases, especially HIV. An early human NK3.3 reporter cell line can be genetically modified to express CD4zeta using a retroviral transduction approach. These CD4zeta-expressing NK cells can specifically kill NK-resistant tumor cells expressing the relevant ligand, HIV envelope glycoprotein 120 (gp120), or CD4+ T cells infected with HIV (Tran et al., 1995). Human NK3.3 cells can be readily activated via CD4zeta-based CARs to target both tumor and virus-infected cells, demonstrating early evidence that CAR-modified NK cells have the potential to be used to treat HIV infection.
Interestingly, a recent study reported that CAR-modified hematopoietic stem/progenitor cells (HSPCs) could differentiate into functional NK cells in humanized mice (Zhen et al., 2015). These NK cells are resistant to HIV infection and suppress HIV replication in vivo. The significance of this study is that CAR-modified HSPCs can differentiate into functional NK cells. This strategy provides a new approach to generate NK cells for immunotherapy. Manipulation of a patient’s immune cells usually presents many challenges. For example, the first problem is that there are insufficient numbers of NK cells that can be obtained from patient’s peripheral blood. The second problem is that NK cells that are freshly isolated from patients usually do not exhibit normal functions, compared with NK cells from healthy individuals. The hematopoietic development of CD4zeta-CAR-expressing NK cells from genetically modified HSPCs can provide a stable, functional, innate immune response to HIV, as a rapid, early immune response is important to control HIV replication. Additionally, CAR-modified HSPCs may possess the ability to produce NK cells over time, since one of the challenges of NK cell-mediated immunotherapy is the short life span of NK cells that are isolated from a patient’s peripheral blood.
In summary, in the field of HIV research, extensive studies have been focused on the roles of T and B cells in HIV infection. An increasing body of evidence indicates the importance of NK cells in HIV infection and HIV disease progression.
THE CAR-MODIFIED NK CELL IS
Adoptive cell-based therapy using CAR-modified NK cells has the potential to extend the survival of cancer patients by enhancing the antitumor effectiveness of CAR-modified cells. In this context, an important question is emerging: How does one efficiently and rapidly choose the best CAR-modified cells for cancer patients? Specifically, researchers from different laboratories are generating different CARs with minor modifications. However, before these CAR-modified cells can enter clinical trials, it is essential that they can be evaluated precisely for their quality and efficacy in a cost-effective manner. Additionally, the speed at which these CAR T cells can be clinically tested is limited by the current, time-consuming, costly, and labor-intensive conventional approaches used to evaluate efficacy. In the field of basic immunology, T cell efficacy is not only controlled by the specificity and avidity of the tumor antigen and T cell interaction, but it also depends on a collective process, involving multiple adhesion and regulatory molecules, spatially organized at the T cell IS.
Here, we review the current progress in CAR-modified T cell and NK cell ISs, with a focus on CAR-NK ISs. The main message in this section is that the NK IS is critical to the understanding of the fundamental mechanisms underlying the cytotoxicity and side effects of CAR-modified T cell- or NK cell-based immunotherapy. Specifically, generation and modification of novel CAR-modified immune cells to target solid cancers and other diseases has been a major effort in the field of immunotherapy. The time has come for scientists to understand the fundamental mechanisms of CAR immunobiology. Lack of such knowledge is an important issue because, without it, choosing the best CAR-modified immune cells for patients with solid tumors or other diseases is highly unlikely.
The background of IS
The NK cell IS (Davis et al., 1999) was first described between peripheral blood NK cells in the YTS cell line and various transfectants of 721.221 (a B cell line derived by mutagenesis that does not express MHC class I molecules (Shimizu et al., 1988)). The cell biology of NK cells and their IS has been reviewed by others (Bromley et al., 2001; Davis, 2002; Lagrue et al., 2013). The original concept of NK IS is derived from T cell IS. Control of T cell activation and modulation of T cell function not only depend on the TCR-epitope-MHC complex interaction, but also on a collective process that involves multiple adhesion and regulatory molecules spatially organized at the T cell-APC interface, forming the T cell IS (Fooksman et al., 2010). Fixed-cell imaging studies on T-cell APC conjugates (Monks et al., 1998) and multiple dynamic studies with planar bilayers (Grakoui et al., 1999; Campi et al., 2005; Varma et al., 2006) have illuminated the molecular organization of physiological T cell activation. The IS corresponds to a concentric structure of discrete domains with TCRs and CD3 molecules occupying the central region (central supramolecular activation cluster; cSMAC), which is surrounded by an outer ring of adhesion molecules in the peripheral SMAC (pSMAC). Besides this classic model largely studied with naïve or resting CD4+ T cells, it has become clear that there are several forms of IS. Effector CD8+ CTL and NK cells can form both cytotoxic IS (leading to killing), stimulatory IS (leading to cytokine secretion), and inhibitory IS (Stinchcombe and Griffiths, 2003; Liu et al., 2009; Liu et al., 2012; Jang et al., 2015). The structure of NK IS appears less organized than T cell IS. For example, the distribution of major IS components, including activation cluster (e.g., CD16) and integrin molecules (e.g., LFA-1), at NK IS formed on the glass-supported planar lipid bilayer containing human IgG1 Fc portion (a ligand for CD16) and ICAM-1 (a ligand for LFA-1) is not well organized (Liu et al., 2009). The effector CTL IS contains a distinct central secretory domain (Stinchcombe et al., 2006), with granule secretion controlled by centrosome delivery to the plasma membrane. Integrity of the pSMAC ring is also important for effective killing (Anikeeva et al., 2005). In summary, basic cytotoxic IS (including CTL and NK cell) structure and molecule pattern can vary, but the function of directed secretion at cytotoxic IS is similar, which leads to killing target cells through the polarized release of lytic granules at IS.
Current approaches for studying IS
Conventional fluorescence microscopy of immune cells represents the most common imaging strategy to investigate the IS (Jang et al., 2015; Zheng et al., 2015a). A high-resolution imaging approach, including electron microscopy (Fig. 1) and fluorescence microscopy in combination with the glass-supported planar lipid bilayer system (Fig. 2), can provide a new look at the structure of the CAR-modified cell IS, allowing a determination of the precise relationship between IS quality and the effectiveness of CAR-modified cells. Unlike Western blot (WB) and immunoprecipitation (IP), which only assess the average signaling state, microscopy-based assays, including the advent of commercially available high-resolution optical microscopes, such as total internal reflection fluorescence (TIRF) microscopy (Liu et al., 2009; Liu et al., 2012) and stimulated emission depletion (STED) microscopy (Zheng et al., 2015a; Zheng et al., 2015b), reveal structure, function, and signaling (i.e., quality) of IS. Also, the IS is one of the most pivotal communication strategies used by immune cells (Jang et al., 2015). In addition to the cell-cell conjugation system, the structure, function, and signaling cascades at the IS have been further confirmed by imaging T cell interactions with a glass-supported, planar lipid bilayer, which contains the MHC-peptide complex and other costimulatory molecules (Tozeren et al., 1992; Grakoui et al., 1999; Lee et al., 2002, 2003; Mossman et al., 2005). The general consensus in the field of immunology is that a glass-supported, planar lipid bilayer system can mimic target cells for the study of the IS at high resolution (Dustin et al., 2007; Choudhuri et al., 2014; Zheng et al., 2015a; Bertolet and Liu, 2016). Previous studies have demonstrated that IS quality determines the efficacy of T cell-mediated cytotoxicity (Grakoui et al., 1999; Jenkins et al., 2009; Dustin and Long, 2010). Similarly, an obvious question is whether CAR-modified T and NK cells can form functional ISs. Indeed, CAR-modified NK cells can form functional ISs on a glass-supported, planar lipid bilayer (Fig. 2), in which the images of fixed CAR-modified NK cells on lipid bilayers reveal the central accumulation of CD19, which is reminiscent of the central cluster of TCRs and B cell receptors at the synapse (Fooksman et al., 2010; Harwood and Batista, 2010). Thus, the glass-supported planar lipid bilayer system can serve as a reductionist approach to study CAR IS. Additionally, CAR T and NK cells do form ISs on the glass-supported lipid bilayer system.
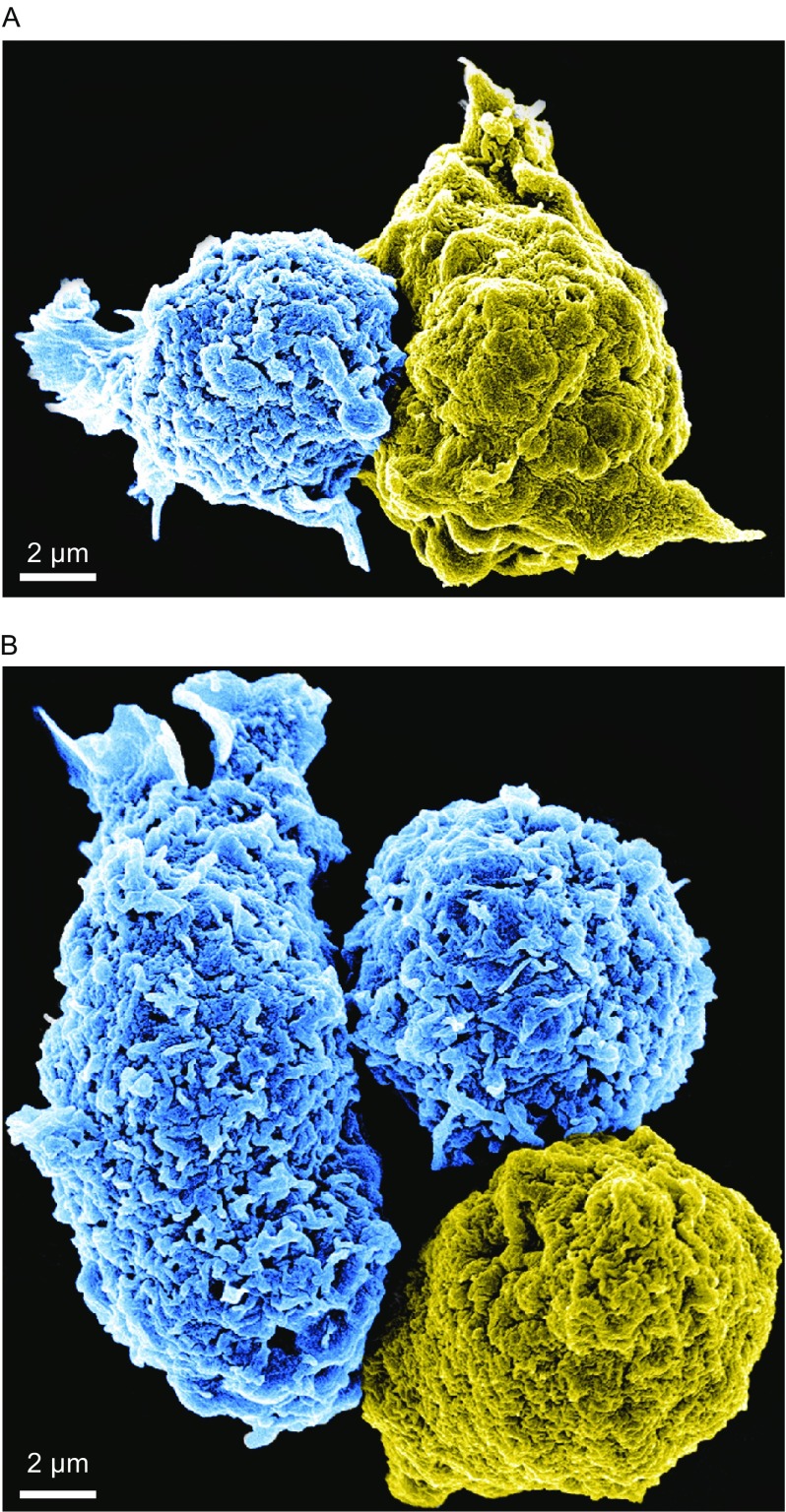
Figure 1.
The CAR-modified NK cell IS. (A) One CAR-modified NK92 cell (blue) interacts with a tumor cell (yellow) through an IS. (B) Two CAR-modified NK92 cells (blue) interact with a tumor cell (yellow) through two different ISs. These are two representative scanning electron micrographs using two different colors
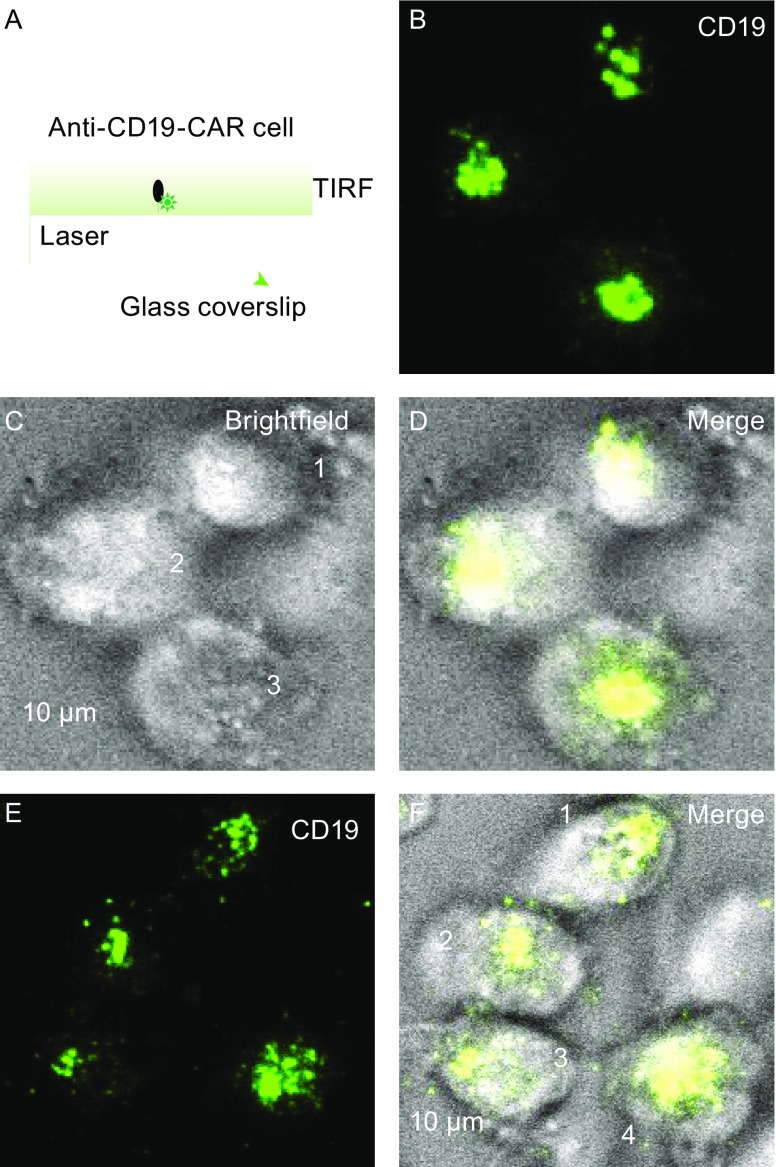
Figure 2.
CAR-modified NK cell ISs on a glass-supported, planar lipid bilayer. (A) Schematic depiction of a TIRF setup in which a lipid bilayer contains a fluorescence dye-labeled tumor antigen (green). TIRF (B), brightfield (C), and merge (D) images of CAR-modified NK cell IS formation on a glass-supported, planar lipid bilayer carrying an Alexa488-labled human CD19 protein (green). The three CAR-modified NK cells that contacted the lipid bilayer, as determined by the central accumulation of tumor antigen under TIRF microscopy, are numbered. Representative TIRF (E) and merge (F) images of CAR-modified NK cells are shown. Four individual CAR-modified NK cells, fixed at 30 min after addition to the bilayer carrying CD19-Alexa Fluor 488, are numbered. The images are representative of at least 100 cells from three independent experiments
The rationale for the investigation of CAR IS
A critical factor affecting the efficacy of NK cells for CAR-modified NK cell-based immunotherapy is the tumor microenvironment, which is a key element in the exploitation of NK cells for the treatment of solid tumors (Gras Navarro et al., 2015). The infiltration of NK cells into solid tumors has been reported previously (Burke et al., 2010; Pietra et al., 2016). The status of tumor-infiltrating NK cells correlates with the prognosis for melanoma (Burke et al., 2010). The interactions between NK cells and the tumor microenvironment include: (1) soluble factor production (transforming growth factor-beta, indoleamine 2,3-dioxygenase, interleukin (IL)-4, prostaglandin E2) by tumor cells or other cells in the tumor microenvironment (Castriconi et al., 2003; Marcenaro et al., 2005); (2) inhibitory cells at the tumor site, which include myeloid-derived suppressor cells, regulatory T cells, tumor-associated macrophages, tumor-associated fibroblasts, and tumor cells (Pietra et al., 2016); and (3) dysfunctional NK cells at the tumor site. These dysfunctional NK cells are characterized by the upregulated expression of several inhibitory receptors, such as PD-1 (Keir et al., 2008; Benson et al., 2010), and the downregulation of critical, stimulatory NK receptors, such as NKG2D (Krockenberger et al., 2008). Among these factors, one of the most important cellular interactions in the tumor microenvironment is the interaction between NK cells and tumor cells, also known as the NK IS (Davis et al., 1999; Davis, 2002; Orange, 2008). A CAR-modified T cell or NK cell must form an effective IS with susceptible target cells to kill them.
Our current knowledge lacks a complete understanding of CAR biology and an effective, unanimously recognized approach to predict the effectiveness of CAR-modified cells. Using the IS quality to predict the efficacy of immunotherapy and side effects of CAR T/NK cells will introduce a superior tool or parameter into the field of immunotherapy. Specifically, to assess the effectiveness of CAR-modified cells, the quality of the IS formed by these cells and susceptible target cells, including virus-infected cells and tumor cells, as well as the glass-supported planar lipid bilayer system for mimicking the surface of an tumor cell or infected target cell, can be quantified.
Traditional biochemical and cell biological approaches for the analysis of signaling pathways (e.g., WB and IP) rely on homogenized cellular extracts from millions of cells. While fast and inexpensive, these methods do not reveal critical spatiotemporal parameters or the dynamics of intracellular signal transduction within the IS.
Although tremendous progress has been made in the basic research of the IS, to date, no study has addressed how the IS controls CAR-modified cell function. Such knowledge is important for optimally choosing the best CAR-modified T cells for cancer patients, as well as a tool to evaluate the efficacy of CAR T/NK cells. Currently available strategies to evaluate the effectiveness of CAR T cells include conventional in vitro methods, such as cytokine secretion, cytotoxicity, proliferation, ratio of CD4/CD8 cells, and long-term killing assays, as well as in vivo mouse models. To assess the homing, persistence, and antitumor activity of CAR T cells in vivo, scientists use a SCID mouse model and the in vivo imaging system (Bhaumik and Gambhir, 2002; Kim et al., 2004; Vera et al., 2006; Wang et al., 2009; Morse and Tannous, 2012). However, currently available in vitro and in vivo analyses are time consuming, costly, and labor intensive; thus, a new approach is urgently needed to solve this problem (Geldres et al., 2016). Accordingly, more sophisticated, high-resolution techniques are warranted to quantitate the function of CAR-modified cells, as described above.
In summary, CAR-modified T cell- and NK cell-mediated ISs are essential to the understanding of the efficacy of CAR-modified T cells and NK cells and their toxicities.
PERSPECTIVES
Recent progress in the understanding of NK cell biology and immunology, including memory NK cells (Adams et al., 2016; Fehniger and Cooper, 2016) and the signaling pathways of immune receptors on NK cells (Long et al., 2013), has established the foundation for harnessing the power of NK cells for innovative immunotherapies. However, there are many potential challenges regarding the use of NK cell-based immunotherapy in the future, as detailed below.
NK cell rapid expansion, cryopreservation, and shipping
Rapid NK cell expansion technique is urgently needed. Recent studies using 4-1BB (also known as CD137) ligand (4-1BBL/CD137L) and IL-21 expressing K562 cells as feeder cells can be used to rapidly expand NK cells in vitro (Denman et al., 2012). However, the characterization and application of these cells for the treatment of patients is essential to ensure that the cells are functional and healthy. In addition, specific NK cell expansion is also needed to advance NK cell immunotherapy in vivo. One potential issue regarding NK cell expansion in vitro using irradiated feeder cells in the presence of cytokine IL-2 is that naïve immune cells become exhausted or senescent after rapid proliferation and differentiation (Keir et al., 2008). Indeed, CAR-modified immune cells express exhaustion markers such as PD-1 (John et al., 2013; Cherkassky et al., 2016; Chong et al., 2016; Gargett et al., 2016). To solve the problem of immune cell exhaustion, one approach is to block PD-1 signaling in CAR-modified T cells (Cherkassky et al., 2016). Another potential strategy is to alter the metabolic pathway in CAR-modified T cells (Ping et al., 2017) or reinforce lymphocyte metabolism (Lim and June, 2017), given the existence of essential metabolic signaling in T cells (Buck et al., 2015). Therefore, it will be of interest to determine whether the alternation of metabolic pathways can enhance NK cell expansion without exhaustion.
At present, the expansion of CAR-modified T and NK cells requires in vitro stimulation of genetically modified T and NK cells using antibodies and cytokines. These antibody and cytokine-driven activation and expansion may negatively alter CAR-T/ NK cell functions. For example, CAR-modified immune cell exhaustion can be induced by the end of extensive expansion program, which is evident by the up-regulation of PD-1, TIM-3, and LAG-3 in CAR T cells (Long et al., 2015). Therefore, new modification and expansion strategies without induction of exhaustion may be developed in vivo, given immune cell exhaustion is a major factor for compromised immune responses against tumor and virus during chronic antigen stimulation (Virgin et al., 2009; Wherry, 2011). Additionally, the current expansion of CAR-modified immune cells for clinical applications takes at least 2–3 weeks, which becomes a significant hurdle for some patients. The “sleeping beauty transposon” or piggBac system, which is capable of delivering large (9.1–14.3 kb) transposable elements without a significant reduction in T cell efficacy (Maiti et al., 2013; Guerrero et al., 2014; Singh et al., 2014), in combination with genetically engineered artificial cells expressing membrane-bound IL-15 and 4-1BB ligands, has already been used for CAR-modified T cell immunotherapy. This approach may promise the rapid expansion of NK cells in the future.
After successful expansion, cryopreservation and transportation of NK cells are also essential to advance NK cell utility in the clinic. After cryopreservation (the freeze/thaw cycle) and transportation, recovered NK cells often experience a decrease in function. The viability of recovered NK cells can also be a potential issue for NK cell-based immunotherapy. Therefore, the development of new cryopreservation and transportation methods is urgently needed.
Sources of NK cells
There are two sources of NK cells (autologous and allogeneic NK cells), which can be obtained from PBMCs, apheresis products, bone marrow, cord blood cells, embryonic stem cells, and induced pluripotent stem cells. Compared to primary NK cells isolated from blood and other sources, human NK92 cell line is easier and affordable. The clinical application of irradiated CAR-NK92 is safe and effective, as the use of NK cell lines can significantly reduce the cost of immunotherapy. Additionally, NK cells directly isolated from immunocompromised cancer patients usually have poor cytotoxicity and functionality, precluding their use. The future development of CAR-modified NK92 products promises to be both feasible and inexpensive.
Strategies for long-lived, expandable NK cells
The life span of NK cells is generally shorter than that of CTLs. Increasing the life span of ex vivo-expanded NK cells has become a pivotal issue in immunotherapy. The advantage of the short life span of CAR-modified NK cells is that they have fewer off-target effects than CAR-modified T cells. Scientists are currently seeking a technique that will expand NK cells in a shorter time period for urgent clinical needs. K562-mbIL21-41BBL cells have recently been used to expand NK cells rapidly (Denman et al., 2012). In the future, it will be essential to produce both long-lived and quickly expandable NK cells, such as memory NK cells (Sun et al., 2011; Adams et al., 2016; Fehniger and Cooper, 2016), for generating CAR-modified NK products.
Potential toxicity of CAR-modified NK cells and manufacturing costs
Generally, it is thought that NK cell-based immunotherapy results in less severe side effects than genetically modified T cell-based immunotherapy. However, a direct comparison of side effects between CAR-modified T cells and NK cells is not available. The routine management of CAR-modified NK cell toxicity is desirable. For example, the use of inducible caspase-9 in the construct (Di Stasi et al., 2011; Sadelain, 2011), causing the dimerization of caspase-9 by FK560, will induce CAR-modified NK cell apoptosis. This strategy limits the potential side effects of CAR-modified NK cells and minimizes other cellular damage, such as that from a cytokine storm. Similarly, the engineered synthetic Notchs (synNotchs) system in CAR-modified T cells (Roybal et al., 2016) may be applicable to CAR-modified NK cells. Furthermore, the current cost of CAR-mediated immunotherapy is high. An FDA-approved, CAR-mediated immunotherapy must undergo multi-phase clinical trials. The approximate costs for phase I, II, and III clinical trials are $2–5, $5–15, and $10–50 million, respectively. Meanwhile, the manufacturing of CAR-modified immune cells is costly, labor intensive, and time consuming. In the future, a closed, automated workflow system is essential to reduce the cost of generating CAR-modified T cells and NK cells.
Enhancement of transfection efficiency for peripheral blood NK cells
One of the biggest obstacles to the use of gene-modified NK cells for immunotherapy has been the absence of an efficient gene transfer technique. Several technologies, including retroviral and lentiviral systems, have been used to enhance the transduction efficiency of NK cell lines and activated, primary NK cells (Lapteva et al., 2016). The subsequent challenge is to apply these approaches to resting NK cells isolated directly from peripheral blood that maintain their cellular functions.
Development of “off-the-shelf” NK cell products
Development of “off-the-shelf” NK cell products is still in the concept stage. There are no universal NK cell products that can be used to treat a variety of tumors. The CAR-modified NK92 cell line may serve as a future “off-the shelf” NK product.
In conclusion, CAR-modified NK cells with manageable toxicities have emerged as a powerful, effective tool for fighting cancer and infectious diseases. To harness the power of these cells, basic research of the cell biology and immunology of CAR-modified NK cells, with a focus on the CAR-modified NK cell-mediated in vitro and in vivo IS, is essential.
ACKNOWLEDGEMENTS
We thank Jianhua (James) Gu (director of the electron microscopy core at Houston Methodist) for the scanning electron micrograph imaging and Matthew G. Landry for the electron microscopy illustration. This work was supported in part by 1R21HL125018-01A1, 1R21AI124769-01, 1R21AI129594-01, 1R56AI130197-01, P50CA126752, the Houston Methodist Career Cornerstone Award, and the Baylor-UT Houston Center for AIDS Research Core Support Grant (number AI36211) from the National Institute of Allergy and Infectious Diseases.
ABBREVIATIONS
ADCC, antibody-dependent cell-mediated cytotoxicity; AML, acute myeloid leukemia; CAR, chimeric antigen receptor; CTLs, cytotoxic T lymphocytes; HLA, human leukocyte antigen; IL, interleukin; IS, immunological synapse or immune synapse; KIR, kill-cell immunoglobulin-like receptor; MHC, major histocompatibility complex; NK, natural killer; PBMCs, peripheral blood mononuclear cells; SCID, severe combined immunodeficiency; scTv, single-chain T cell receptor variable fragment; T-ALL, T cell acute lymphoblastic leukemia; TCR, T cell receptor; TIRF, total internal reflection fluorescence
COMPLIANCE WITH ETHICS GUIDELINES
Dongfang Liu, Shuo Tian, Kai Zhang, Wei Xiong, Ndongala Michel Lubaki, Zhiying Chen, and Weidong Han declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Footnotes
An erratum to this article is available at https://doi.org/10.1007/s13238-017-0427-1.
Contributor Information
Dongfang Liu, Email: dliu2@houstonmethodist.org.
Weidong Han, Email: hanwdrsw69@yahoo.com.
REFERENCES
- Ada G. Twenty years into the saga of MHC-restriction. Immunol Cell Biol. 1994;72:447–454. doi: 10.1038/icb.1994.68. [DOI] [PubMed] [Google Scholar]
- Adams NM, O’Sullivan TE, Geary CD, Karo JM, Amezquita RA, Joshi NS, Kaech SM, Sun JC. NK cell responses redefine immunological memory. J Immunol. 2016;197:2963–2970. doi: 10.4049/jimmunol.1600973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggen DH, Chervin AS, Schmitt TM, Engels B, Stone JD, Richman SA, Piepenbrink KH, Baker BM, Greenberg PD, Schreiber H, et al. Single-chain Valpha Vbeta T-cell receptors function without mispairing with endogenous TCR chains. Gene Ther. 2012;19:365–374. doi: 10.1038/gt.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akl MR, Nagpal P, Ayoub NM, Prabhu SA, Gliksman M, Tai B, Hatipoglu A, Goy A, Suh KS. Molecular and clinical profiles of syndecan-1 in solid and hematological cancer for prognosis and precision medicine. Oncotarget. 2015;6:28693–28715. doi: 10.18632/oncotarget.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Kitchen SG, Chen IS, Ng HL, Zack JA, Yang OO. HIV-1-specific chimeric antigen receptors based on broadly neutralizing antibodies. J Virol. 2016;90:6999–7006. doi: 10.1128/JVI.00805-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Altfeld M. NK cell function in HIV-1 infection. Curr Mol Med. 2006;6:621–629. doi: 10.2174/156652406778195035. [DOI] [PubMed] [Google Scholar]
- Alter G, Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J Intern Med. 2009;265:29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Altfeld M. Mutiny or scrutiny: NK cell modulation of DC function in HIV-1 infection. Trends Immunol. 2011;32:219–224. doi: 10.1016/j.it.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, Streeck H, Waring M, Meier A, Brander C, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI, et al. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011;476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altfeld M, Gale M., Jr Innate immunity against HIV-1 infection. Nat Immunol. 2015;16:554–562. doi: 10.1038/ni.3157. [DOI] [PubMed] [Google Scholar]
- Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11:176–186. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:6437–6442. doi: 10.1073/pnas.0502467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, Klingemann H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–632. doi: 10.1080/14653240802301872. [DOI] [PubMed] [Google Scholar]
- Bakker AB, Wu J, Phillips JH, Lanier LL. NK cell activation: distinct stimulatory pathways counterbalancing inhibitory signals. Hum Immunol. 2000;61:18–27. doi: 10.1016/S0198-8859(99)00160-3. [DOI] [PubMed] [Google Scholar]
- Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein HB, Wang G, Plasterer MC, Zack JA, Ramasastry P, Mumenthaler SM, Kitchen CM. CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function. Virology. 2009;387:59–66. doi: 10.1016/j.virol.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolet G, Liu D. The planar lipid bilayer system serves as a reductionist approach for studying NK cell immunological synapses and their functions. Methods Mol Biol. 2016;1441:151–165. doi: 10.1007/978-1-4939-3684-7_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik S, Gambhir SS. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci U S A. 2002;99:377–382. doi: 10.1073/pnas.012611099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Boissel L, Betancur-Boissel M, Lu W, Krause DS, Van Etten RA, Wels WS, Klingemann H. Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology. 2013;2:e26527. doi: 10.4161/onci.26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges da Silva H, Fonseca R, Alvarez JM, D’Imperio Lima MR. IFN-gamma priming effects on the maintenance of effector memory CD4(+) T cells and on phagocyte function: evidences from infectious diseases. J Immunol Res. 2015;2015:202816. doi: 10.1155/2015/202816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, Ljunggren HG. Tumor cell recognition by the NK cell activating receptor NKG2D. Eur J Immunol. 2008;38:2957–2961. doi: 10.1002/eji.200838833. [DOI] [PubMed] [Google Scholar]
- Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke S, Lakshmikanth T, Colucci F, Carbone E. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol. 2010;31:339–345. doi: 10.1016/j.it.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Campbell KS, Yusa S, Kikuchi-Maki A, Catina TL. NKp44 triggers NK cell activation through DAP12 association that is not influenced by a putative cytoplasmic inhibitory sequence. J Immunol. 2004;172:899–906. doi: 10.4049/jimmunol.172.2.899. [DOI] [PubMed] [Google Scholar]
- Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr WH, Rosen DB, Arase H, Nixon DF, Michaelsson J, Lanier LL. Cutting edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation. J Immunol. 2007;178:647–651. doi: 10.4049/jimmunol.178.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A. 2003;100:4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehimi J, Bandyopadhyay S, Prakash K, Perussia B, Hassan NF, Kawashima H, Campbell D, Kornbluth J, Starr SE. In vitro infection of natural killer cells with different human immunodeficiency virus type 1 isolates. J Virol. 1991;65:1812–1822. doi: 10.1128/jvi.65.4.1812-1822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Han J, Chu J, Zhang L, Zhang J, Chen C, Chen L, Wang Y, Wang H, Yi L, et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget. 2016;7:27764–27777. doi: 10.18632/oncotarget.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Wada M, Pinz KG, Liu H, Lin KW, Jares A, Firor AE, Shuai X, Salman H, Golightly M, et al. Preclinical targeting of aggressive T cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia. 2017 doi: 10.1038/leu.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho FN, Chang TH, Shu CW, Ko MC, Liao SK, Wu KH, Yu MS, Lin SJ, Hong YC, Chen CH, et al. Enhanced cytotoxicity of natural killer cells following the acquisition of chimeric antigen receptors through trogocytosis. PLoS ONE. 2014;9:e109352. doi: 10.1371/journal.pone.0109352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, Crotty S. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol. 2013;190:4014–4026. doi: 10.4049/jimmunol.1202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine B, June CH, Schuster SJ. PD-1 blockade modulates chimeric antigen receptor (CAR) modified T cells and induces tumor regression: refueling the CAR. Blood. 2016;129(8):1039–1041. doi: 10.1182/blood-2016-09-738245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri K, Llodra J, Roth EW, Tsai J, Gordo S, Wucherpfennig KW, Kam LC, Stokes DL, Dustin ML. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507:118–123. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, Peng Y, Mao H, Yi L, Ghoshal K, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28:917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A, Rollman E, Johansson S, Kent SJ, Stratov I. The utility of ADCC responses in HIV infection. Curr HIV Res. 2008;6:515–519. doi: 10.2174/157016208786501472. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol. 2016;14:55–60. doi: 10.1038/nrmicro.2015.5. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/S1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- Cong L, Zhang F. Genome engineering using CRISPR-Cas9 system. Methods Mol Biol. 2015;1239:197–217. doi: 10.1007/978-1-4939-1862-1_10. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99:3661–3667. doi: 10.1182/blood.V99.10.3661. [DOI] [PubMed] [Google Scholar]
- Curti A, Ruggeri L, D’Addio A, Bontadini A, Dan E, Motta MR, Trabanelli S, Giudice V, Urbani E, Martinelli G, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–3279. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- Davis DM. Assembly of the immunological synapse for T cells and NK cells. Trends Immunol. 2002;23:356–363. doi: 10.1016/S1471-4906(02)02243-3. [DOI] [PubMed] [Google Scholar]
- Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL. The human natural killer cell immune synapse. Proc Natl Acad Sci U S A. 1999;96:15062–15067. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maria A, Moretta L. NK cell function in HIV-1 infection. Curr HIV Res. 2008;6:433–440. doi: 10.2174/157016208785861221. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, Chun TW, Churchill M, Di Mascio M, Katlama C, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12:607–614. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Chiesa M, Romeo E, Falco M, Balsamo M, Augugliaro R, Moretta L, Bottino C, Moretta A, Vitale M. Evidence that the KIR2DS5 gene codes for a surface receptor triggering natural killer cell function. Eur J Immunol. 2008;38:2284–2289. doi: 10.1002/eji.200838434. [DOI] [PubMed] [Google Scholar]
- Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, Singh H, Hurton L, Maiti SN, Huls MH, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, Jemal A. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016 doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Long EO. Cytotoxic immunological synapses. Immunol Rev. 2010;235:24–34. doi: 10.1111/j.0105-2896.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Starr T, Varma R, Thomas VK. Unit 18 13 Supported planar bilayers for study of the immunological synapse. Curr Protoc Immunol. 2007 doi: 10.1002/0471142735.im1813s76. [DOI] [PubMed] [Google Scholar]
- Esser R, Muller T, Stefes D, Kloess S, Seidel D, Gillies SD, Aperlo-Iffland C, Huston JS, Uherek C, Schonfeld K, et al. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16:569–581. doi: 10.1111/j.1582-4934.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda L, Alter G. KIR/HLA: genetic clues for a role of NK cells in the control of HIV. Adv Exp Med Biol. 2011;780:27–36. doi: 10.1007/978-1-4419-5632-3_3. [DOI] [PubMed] [Google Scholar]
- Farnault L, Sanchez C, Baier C, Le Treut T, Costello RT. Hematological malignancies escape from NK cell innate immune surveillance: mechanisms and therapeutic implications. Clin Dev Immunol. 2012;2012:421702. doi: 10.1155/2012/421702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Cooper MA. Harnessing NK cell memory for cancer immunotherapy. Trends Immunol. 2016;37:877–888. doi: 10.1016/j.it.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristan C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke J, Durr R, Dietrich U, Koch J. Natural killer cells in HIV-1 infection: a double-edged sword. AIDS Rev. 2011;13:67–76. [PubMed] [Google Scholar]
- Gargett T, Yu W, Dotti G, Yvon ES, Christo SN, Hayball JD, Lewis ID, Brenner MK, Brown MP. GD2-specific CAR T Cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol Ther. 2016;24:1135–1149. doi: 10.1038/mt.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldres C, Savoldo B, Dotti G. Chimeric antigen receptor-redirected T cells return to the bench. Semin Immunol. 2016;28(1):3–9. doi: 10.1016/j.smim.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med. 2012;18:377–384. doi: 10.1016/j.molmed.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev. 2015;263:68–89. doi: 10.1111/imr.12243. [DOI] [PubMed] [Google Scholar]
- Gill S, Maus MV, Porter DL. Chimeric antigen receptor T cell therapy: 25years in the making. Blood Rev. 2016;30:157–167. doi: 10.1016/j.blre.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, Spellman S, Haagenson MD, Lenvik AJ, Litzow MR, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123:3016–3026. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, Grez M, Kloess S, Arseniev L, Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol. 2015;6:21. doi: 10.3389/fphar.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–658. [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Gras Navarro A, Bjorklund AT, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol. 2015;6:202. doi: 10.3389/fimmu.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero AD, Moyes JS, Cooper LJ. The human application of gene therapy to re-program T-cell specificity using chimeric antigen receptors. Chin J Cancer. 2014;33:421–433. doi: 10.5732/cjc.014.10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, Victor A, Meisen WH, Kim SH, Grandi P, et al. CAR-engineered NK Cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep. 2015;5:11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood NE, Batista FD. Early events in B cell activation. Annu Rev Immunol. 2010;28:185–210. doi: 10.1146/annurev-immunol-030409-101216. [DOI] [PubMed] [Google Scholar]
- Hatano H, Jain V, Hunt PW, Lee TH, Sinclair E, Do TD, Hoh R, Martin JN, McCune JM, Hecht F, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2013;208:50–56. doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley M, Bourbigot S, Booth V. Self-association of an activating natural killer cell receptor, KIR2DS1. PLoS ONE. 2011;6:e23052. doi: 10.1371/journal.pone.0023052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hens J, Jennes W, Kestens L. The role of NK cells in HIV-1 protection: autologous, allogeneic or both? AIDS Res Ther. 2016;13:15. doi: 10.1186/s12981-016-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16:230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Hermanson DL, Kaufman DS. Utilizing chimeric antigen receptors to direct natural killer cell activity. Front Immunol. 2015;6:195. doi: 10.3389/fimmu.2015.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14:2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello A, Debbeche O, Samarani S, Ahmad A. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J Leukoc Biol. 2008;84:1–26. doi: 10.1189/jlb.0907650. [DOI] [PubMed] [Google Scholar]
- Iannello A, Debbeche O, Samarani S, Ahmad A. Antiviral NK cell responses in HIV infection: II. Viral strategies for evasion and lessons for immunotherapy and vaccination. J Leukoc Biol. 2008;84:27–49. doi: 10.1189/jlb.0907649. [DOI] [PubMed] [Google Scholar]
- Jang JH, Huang Y, Zheng P, Jo MC, Bertolet G, Zhu MX, Qin L, Liu D. Imaging of cell-cell communication in a vertical orientation reveals high-resolution structure of immunological synapse and novel PD-1 dynamics. J Immunol. 2015;195:1320–1330. doi: 10.4049/jimmunol.1403143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MR, Tsun A, Stinchcombe JC, Griffiths GM. The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse. Immunity. 2009;31:621–631. doi: 10.1016/j.immuni.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Zhang W, Shang P, Zhang H, Fu W, Ye F, Zeng T, Huang H, Zhang X, Sun W, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Mol Oncol. 2014;8:297–310. doi: 10.1016/j.molonc.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John LB, Kershaw MH, Darcy PK. Blockade of PD-1 immunosuppression boosts CAR T-cell therapy. Oncoimmunology. 2013;2:e26286. doi: 10.4161/onci.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Walker BD. HIV-specific CD8(+) T cells and HIV eradication. J Clin Invest. 2016;126:455–463. doi: 10.1172/JCI80566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost S, Altfeld M. Evasion from NK cell-mediated immune responses by HIV-1. Microbes Infect. 2012;14:904–915. doi: 10.1016/j.micinf.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–194. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw MH, Teng MW, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5:928–940. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Dubey P, Ray P, Gambhir SS, Witte ON. Multimodality imaging of lymphocytic migration using lentiviral-based transduction of a tri-fusion reporter gene. Mol Imaging Biol. 2004;6:331–340. doi: 10.1016/j.mibio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Kim MG, Kim D, Suh SK, Park Z, Choi MJ, Oh YK. Current status and regulatory perspective of chimeric antigen receptor-modified T cell therapeutics. Arch Pharm Res. 2016;39:437–452. doi: 10.1007/s12272-016-0719-7. [DOI] [PubMed] [Google Scholar]
- Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy - advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016;7:91. doi: 10.3389/fimmu.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramski M, Parsons MS, Stratov I, Kent SJ. HIV-specific antibody immunity mediated through NK cells and monocytes. Curr HIV Res. 2013;11:388–406. doi: 10.2174/1570162X113116660061. [DOI] [PubMed] [Google Scholar]
- Krockenberger M, Dombrowski Y, Weidler C, Ossadnik M, Honig A, Hausler S, Voigt H, Becker JC, Leng L, Steinle A, et al. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J Immunol. 2008;180:7338–7348. doi: 10.4049/jimmunol.180.11.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrue K, Carisey A, Oszmiana A, Kennedy PR, Williamson DJ, Cartwright A, Barthen C, Davis DM. The central role of the cytoskeleton in mechanisms and functions of the NK cell immune synapse. Immunol Rev. 2013;256:203–221. doi: 10.1111/imr.12107. [DOI] [PubMed] [Google Scholar]
- Lam VC, Lanier LL. NK cells in host responses to viral infections. Curr Opin Immunol. 2016;44:43–51. doi: 10.1016/j.coi.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. doi: 10.1016/S1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- Lapteva N, Parihar R, Rollins LA, Gee AP, Rooney CM. Large-scale culture and genetic modification of human natural killer cells for cellular therapy. Methods Mol Biol. 2016;1441:195–202. doi: 10.1007/978-1-4939-3684-7_16. [DOI] [PubMed] [Google Scholar]
- Le Bert N, Gasser S. Advances in NKG2D ligand recognition and responses by NK cells. Immunol Cell Biol. 2014;92:230–236. doi: 10.1038/icb.2013.111. [DOI] [PubMed] [Google Scholar]
- Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisovsky I, Isitman G, Song R, DaFonseca S, Tremblay-McLean A, Lebouche B, Routy JP, Bruneau J, Bernard NF. A higher frequency of NKG2A+ than of NKG2A- NK cells responds to autologous HIV-infected CD4 cells irrespective of whether or not they coexpress KIR3DL1. J Virol. 2015;89:9909–9919. doi: 10.1128/JVI.01546-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Bryceson YT, Meckel T, Vasiliver-Shamis G, Dustin ML, Long EO. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 2009;31:99–109. doi: 10.1016/j.immuni.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Peterson ME, Long EO. The adaptor protein Crk controls activation and inhibition of natural killer cells. Immunity. 2012;36:600–611. doi: 10.1016/j.immuni.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Patel B, Ghanem MH, Bundoc V, Zheng Z, Morgan RA, Rosenberg SA, Dey B, Berger EA. Novel CD4-based bispecific chimeric antigen receptor designed for enhanced anti-HIV potency and absence of HIV entry receptor activity. J Virol. 2015;89:6685–6694. doi: 10.1128/JVI.00474-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zou F, Lu L, Chen C, He D, Zhang X, Tang X, Liu C, Li L, Zhang H. Chimeric antigen receptor T cells guided by the single-chain Fv of a broadly neutralizing antibody specifically and effectively eradicate virus reactivated from latency in CD4+ T lymphocytes isolated from HIV-1-infected individuals receiving suppressive combined antiretroviral therapy. J Virol. 2016;90:9712–9724. doi: 10.1128/JVI.00852-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Pan Y, Meng S, Zhang W, Zhou F. Current treatment options of T cell-associated immunotherapy in multiple myeloma. Clin Exp Med. 2017 doi: 10.1007/s10238-017-0450-9. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Cheng C, Cheng AW, Zhang X, Li N, Xia C, Wei X, Liu X, Wang H. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 2017;27:154–157. doi: 10.1038/cr.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-S. [DOI] [PubMed] [Google Scholar]
- Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long BR, Ndhlovu LC, Oksenberg JR, Lanier LL, Hecht FM, Nixon DF, Barbour JD. Conferral of enhanced natural killer cell function by KIR3DS1 in early human immunodeficiency virus type 1 infection. J Virol. 2008;82:4785–4792. doi: 10.1128/JVI.02449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetkens T, Yousef S, Radhakrishnan SV, Atanackovic D. Current strategies for the immunotherapy of multiple myeloma. Oncology (Williston Park) 2017;31(1):55–63. [PubMed] [Google Scholar]
- Magana-Maldonado R, Chavez-Cortez EG, Olascoaga-Arellano NK, Lopez-Mejia M, Maldonado-Leal FM, Sotelo J, Pineda B. Immunological evasion in glioblastoma. Biomed Res Int. 2016;2016:7487313. doi: 10.1155/2016/7487313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti SN, Huls H, Singh H, Dawson M, Figliola M, Olivares S, Rao P, Zhao YJ, Multani A, Yang G, et al. Sleeping beauty system to redirect T-cell specificity for human applications. J Immunother. 2013;36:112–123. doi: 10.1097/CJI.0b013e3182811ce9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcenaro E, Della Chiesa M, Bellora F, Parolini S, Millo R, Moretta L, Moretta A. IL-12 or IL-4 prime human NK cells to mediate functionally divergent interactions with dendritic cells or tumors. J Immunol. 2005;174:3992–3998. doi: 10.4049/jimmunol.174.7.3992. [DOI] [PubMed] [Google Scholar]
- Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, Weiss WA. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078. doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- Maude S, Barrett DM. Current status of chimeric antigen receptor therapy for haematological malignancies. Br J Haematol. 2016;172:11–22. doi: 10.1111/bjh.13792. [DOI] [PubMed] [Google Scholar]
- Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, Marcenaro E, Bottino C, Moretta L, Moretta A, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Morse D, Tannous BA. A water-soluble coelenterazine for sensitive in vivo imaging of coelenterate luciferases. Mol Ther. 2012;20:692–693. doi: 10.1038/mt.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16:7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310:1191–1193. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- Mzingwane ML, Tiemessen CT. Mechanisms of HIV persistence in HIV reservoirs. Rev Med Virol. 2017 doi: 10.1002/rmv.1924. [DOI] [PubMed] [Google Scholar]
- Oelsner S, Friede ME, Zhang C, Wagner J, Badura S, Bader P, Ullrich E, Ottmann OG, Klingemann H, Tonn T, et al. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy. 2017;19:235–249. doi: 10.1016/j.jcyt.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Lanier LL. NKG2D in NK and T cell-mediated immunity. J Clin Immunol. 2005;25:534–540. doi: 10.1007/s10875-005-8786-4. [DOI] [PubMed] [Google Scholar]
- Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS (2013) Natural killer cell deficiency. J Allergy Clin Immunol 132, 515–525; quiz 526. [DOI] [PMC free article] [PubMed]
- Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD, Investigators HIVOS. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- Pallikkuth S, Sharkey M, Babic DZ, Gupta S, Stone GW, Fischl MA, Stevenson M, Pahwa S. Peripheral T follicular helper cells are the major HIV reservoir within central memory CD4 T cells in peripheral blood from chronic HIV infected individuals on cART. J Virol. 2015;90(6):2718–2728. doi: 10.1128/JVI.02883-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal V, Yamada E, Martin MP, Alter G, Altfeld M, Metcalf JA, Baseler MW, Adelsberger JW, Carrington M, Anderson SK, et al. Detection of KIR3DS1 on the cell surface of peripheral blood NK cells facilitates identification of a novel null allele and assessment of KIR3DS1 expression during HIV-1 infection. J Immunol. 2007;179:1625–1633. doi: 10.4049/jimmunol.179.3.1625. [DOI] [PubMed] [Google Scholar]
- Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietra G, Vitale C, Pende D, Bertaina A, Moretta F, Falco M, Vacca P, Montaldo E, Cantoni C, Mingari MC, et al. Human natural killer cells: news in the therapy of solid tumors and high-risk leukemias. Cancer Immunol Immunother. 2016;65:465–476. doi: 10.1007/s00262-015-1744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Y, Liu C, Zhang Y. T-cell receptor-engineered T cells for cancer treatment: current status and future directions. Protein Cell. 2017 doi: 10.1007/s13238-016-0367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani K, Rouce RH. The application of natural killer cell immunotherapy for the treatment of cancer. Front Immunol. 2015;6:578. doi: 10.3389/fimmu.2015.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood. 2010;115:1354–1363. doi: 10.1182/blood-2009-08-237370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol. 1996;24:406–415. [PubMed] [Google Scholar]
- Rosenberg EB, McCoy JL, Green SS, Donnelly FC, Siwarski DF, Levine PH, Herberman RB. Destruction of human lymphoid tissue-culture cell lines by human peripheral lymphocytes in 51Cr-release cellular cytotoxicity assays. J Natl Cancer Inst. 1974;52:345–352. doi: 10.1093/jnci/52.2.345. [DOI] [PubMed] [Google Scholar]
- Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164:770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydyznski C, Daniels KA, Karmele EP, Brooks TR, Mahl SE, Moran MT, Li C, Sutiwisesak R, Welsh RM, Waggoner SN. Generation of cellular immune memory and B-cell immunity is impaired by natural killer cells. Nat Commun. 2015;6:6375. doi: 10.1038/ncomms7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain M. Eliminating cells gone astray. N Engl J Med. 2011;365:1735–1737. doi: 10.1056/NEJMe1109971. [DOI] [PubMed] [Google Scholar]
- Schonfeld K, Sahm C, Zhang C, Naundorf S, Brendel C, Odendahl M, Nowakowska P, Bonig H, Kohl U, Kloess S, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther. 2015;23:330–338. doi: 10.1038/mt.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Algara D, Vuillier F, Cayota A, Rame V, Guetard D, Moncany ML, Marasescu M, Dauguet C, Dighiero G. In vitro non-productive infection of purified natural killer cells by the BRU isolate of the human immunodeficiency virus type 1. J Gen Virol. 1993;74(Pt 4):725–731. doi: 10.1099/0022-1317-74-4-725. [DOI] [PubMed] [Google Scholar]
- Scully E, Alter G. NK cells in HIV disease. Curr HIV/AIDS Rep. 2016;13:85–94. doi: 10.1007/s11904-016-0310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Geraghty DE, Koller BH, Orr HT, DeMars R. Transfer and expression of three cloned human non-HLA-A, B, C class I major histocompatibility complex genes in mutant lymphoblastoid cells. Proc Natl Acad Sci U S A. 1988;85:227–231. doi: 10.1073/pnas.85.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Siliciano RF. Opening fronts in HIV vaccine development: targeting reservoirs to clear and cure. Nat Med. 2014;20:480–481. doi: 10.1038/nm.3550. [DOI] [PubMed] [Google Scholar]
- Singh H, Huls H, Kebriaei P, Cooper LJ. A new approach to gene therapy using sleeping beauty to genetically modify clinical-grade T cells to target CD19. Immunol Rev. 2014;257:181–190. doi: 10.1111/imr.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KM, Wu J, Bakker AB, Phillips JH, Lanier LL. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J Immunol. 1998;161:7–10. [PubMed] [Google Scholar]
- Stinchcombe JC, Griffiths GM. The role of the secretory immunological synapse in killing by CD8+ CTL. Semin Immunol. 2003;15:301–305. doi: 10.1016/j.smim.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–1337. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J Natl Cancer Inst. 1980;65:929–935. [PubMed] [Google Scholar]
- Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–1570. doi: 10.1016/j.jcyt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Topfer K, Cartellieri M, Michen S, Wiedemuth R, Muller N, Lindemann D, Bachmann M, Fussel M, Schackert G, Temme A. DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J Immunol. 2015;194:3201–3212. doi: 10.4049/jimmunol.1400330. [DOI] [PubMed] [Google Scholar]
- Torre LA, Sauer AM, Chen MS, Jr, Kagawa-Singer M, Jemal A, Siegel RL. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: converging incidence in males and females. CA Cancer J Clin. 2016 doi: 10.3322/caac.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozeren A, Sung KL, Sung LA, Dustin ML, Chan PY, Springer TA, Chien S. Micromanipulation of adhesion of a Jurkat cell to a planar bilayer membrane containing lymphocyte function-associated antigen 3 molecules. J Cell Biol. 1992;116:997–1006. doi: 10.1083/jcb.116.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran AC, Zhang D, Byrn R, Roberts MR. Chimeric zeta-receptors direct human natural killer (NK) effector function to permit killing of NK-resistant tumor cells and HIV-infected T lymphocytes. J Immunol. 1995;155:1000–1009. [PubMed] [Google Scholar]
- Travar M, Petkovic M, Verhaz A. Type I, II, and III interferons: regulating immunity to mycobacterium tuberculosis Infection. Arch Immunol Ther Exp (Warsz) 2016;64:19–31. doi: 10.1007/s00005-015-0365-7. [DOI] [PubMed] [Google Scholar]
- Valentin A, Rosati M, Patenaude DJ, Hatzakis A, Kostrikis LG, Lazanas M, Wyvill KM, Yarchoan R, Pavlakis GN. Persistent HIV-1 infection of natural killer cells in patients receiving highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2002;99:7015–7020. doi: 10.1073/pnas.102672999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, Wu J, Heslop HE, Rooney CM, Brenner MK, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinuesa CG. HIV and T follicular helper cells: a dangerous relationship. J Clin Invest. 2012;122:3059–3062. doi: 10.1172/JCI65175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Reighard SD, Gyurova IE, Cranert SA, Mahl SE, Karmele EP, McNally JP, Moran MT, Brooks TR, Yaqoob F, et al. Roles of natural killer cells in antiviral immunity. Curr Opin Virol. 2016;16:15–23. doi: 10.1016/j.coviro.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF, Wang HY. Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res. 2017;27:11–37. doi: 10.1038/cr.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cao F, De A, Cao Y, Contag C, Gambhir SS, Wu JC, Chen X. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells. 2009;27:1548–1558. doi: 10.1002/stem.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, Mavilio D, Planelles V, Barker E. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog. 2009;5:e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzl C, Long EO. Unit 11 19B Signal transduction during activation and inhibition of natural killer cells. Curr Protoc Immunol. 2010 doi: 10.1002/0471142735.im1109bs90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Yagita M, Huang CL, Umehara H, Matsuo Y, Tabata R, Miyake M, Konaka Y, Takatsuki K. A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia. 2000;14:922–930. doi: 10.1038/sj.leu.2401769. [DOI] [PubMed] [Google Scholar]
- Yang OO, Tran AC, Kalams SA, Johnson RP, Roberts MR, Walker BD. Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells. Proc Natl Acad Sci U S A. 1997;94:11478–11483. doi: 10.1073/pnas.94.21.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama WM. Mistaken notions about natural killer cells. Nat Immunol. 2008;9:481–485. doi: 10.1038/ni1583. [DOI] [PubMed] [Google Scholar]
- Zhang C, Burger MC, Jennewein L, Genssler S, Schonfeld K, Zeiner P, Hattingen E, Harter PN, Mittelbronn M, Tonn T, et al. ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J Natl Cancer Inst. 2016 doi: 10.1093/jnci/djv375. [DOI] [PubMed] [Google Scholar]
- Zhen A, Kamata M, Rezek V, Rick J, Levin B, Kasparian S, Chen IS, Yang OO, Zack JA, Kitchen SG. HIV-specific immunity derived from chimeric antigen receptor-engineered stem cells. Mol Ther. 2015;23:1358–1367. doi: 10.1038/mt.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Bertolet G, Chen Y, Huang S, and Liu D (2015a) Super-resolution imaging of the natural killer cell immunological synapse on a glass-supported planar lipid bilayer. J Vis Exp [DOI] [PMC free article] [PubMed]
- Zheng P, Noroski LM, Hanson IC, Chen Y, Lee ME, Huang Y, Zhu MX, Banerjee PP, Makedonas G, Orange JS, et al. Molecular mechanisms of functional natural killer deficiency in patients with partial DiGeorge syndrome. J Allergy Clin Immunol. 2015;135:1293–1302. doi: 10.1016/j.jaci.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]