Table 2.
Palladium-catalyzed phenoxazine ether derivatives formation from phenols(a).
| Entry | Phenoxazine compounds | Phenols | Products | Reaction time (h) | % yields (%) |
|---|---|---|---|---|---|
| 1 | 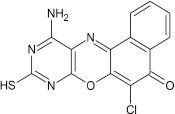 |
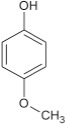 |
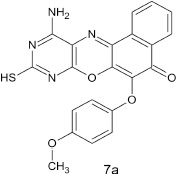 |
8 | 96 |
| 2 | 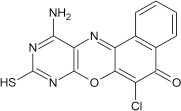 |
 |
 |
10 | 96 |
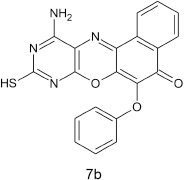
| 3 | 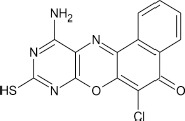 |
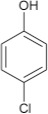 |
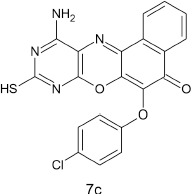 |
8 | 74 |
| 4 | 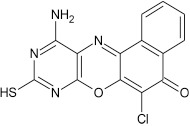 |
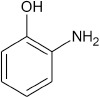 |
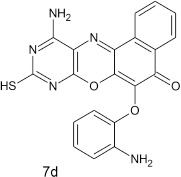 |
10 | 91 |
| 5 | 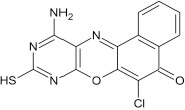 |
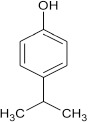 |
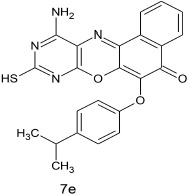 |
7 | 96 |
| 6 | 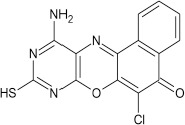 |
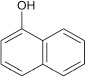 |
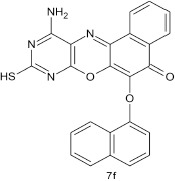 |
10 | 99 |
| 7 | 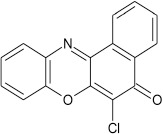 |
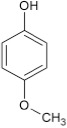 |
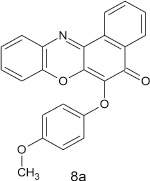 |
8 | 97 |
| 8 | 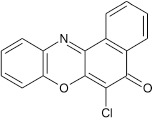 |
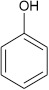 |
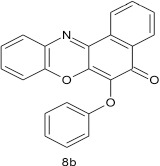 |
10 | 95 |
| 9 | 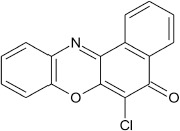 |
 |
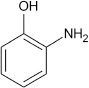
 |
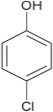
8 | 46 |
| 10 | 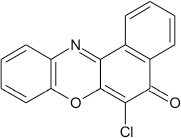 |
 |
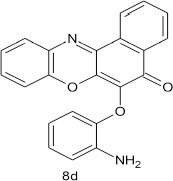 |
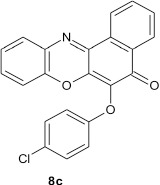
10 | 94 |
| 11 | 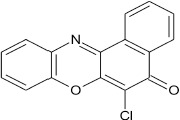 |
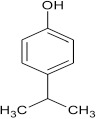 |
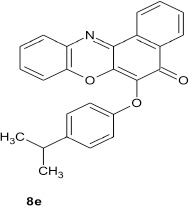 |
7 | 98 |
| 12 | 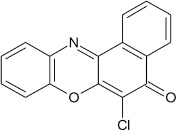 |
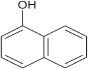 |
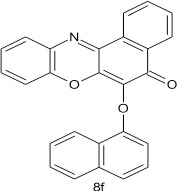 |
10 | 68 |
(a) Reaction conditions: angular phenoxazine (1.0 equiv), phenol (1.2 equiv), K3PO4 (2.0 equiv), ligand (3.0 mol%), toluene (0.3mL), Reflux at 100–110°C, 6–10 h, palladium acetate (4.5 mg); Ligand used: 2-(di-ter-butylphosphino)biphenyl (t-BuXphos).
