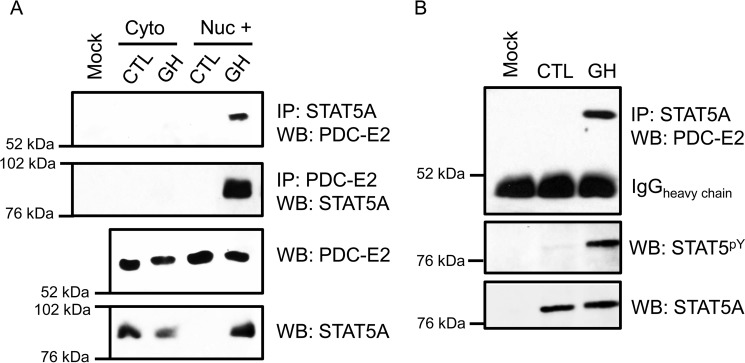
Figure 1.
PDC-E2 interacts with STAT5A in GH-stimulated murine and human adipocytes. Fully differentiated 3T3-L1 adipocytes (A) or human adipocytes (B) were treated with 5 nm mGH (A) or hGH (B) for 15–20 min. Control (CTL) cells were untreated. A, monolayers were collected and subjected to subcellular fractionation that was optimized to separate cytoplasmic (cyto) and nuclear (nuc) compartments. The “Nuc +” fraction was free from cytoplasmic contamination and enriched for nuclei. Immunoprecipitation (IP) reactions utilized anti-STAT5A or anti-PDC-E2 antibodies and contained 300 μg of total protein per reaction. Interacting PDC-E2 (70 kDa) and STAT5A (95 kDa) proteins were detected by WB. Mock samples contained IP antibody but no extract. In the bottom two panels, the presence of STAT5A and PDC-E2 in the extracts used for immunoprecipitation was examined directly by Western blotting using 50 μg of total protein per lane. Experiments using 3T3-L1 adipocytes have been repeated more than three times on independent batches of cells. B, fully differentiated human adipocytes, derived from preadipocytes isolated from the visceral omental adipose depot of obese individuals, were purchased from Zenbio. Monolayers were collected, and whole-cell lysates were prepared. IP reactions and Western blotting were performed as described for A, except that 75 μg of total protein was used for each IP. The middle panel in B demonstrates the presence of activated STAT5 phosphorylated at tyrosine 694/699 (STAT5pY). The experiment with human adipocytes was performed one time.